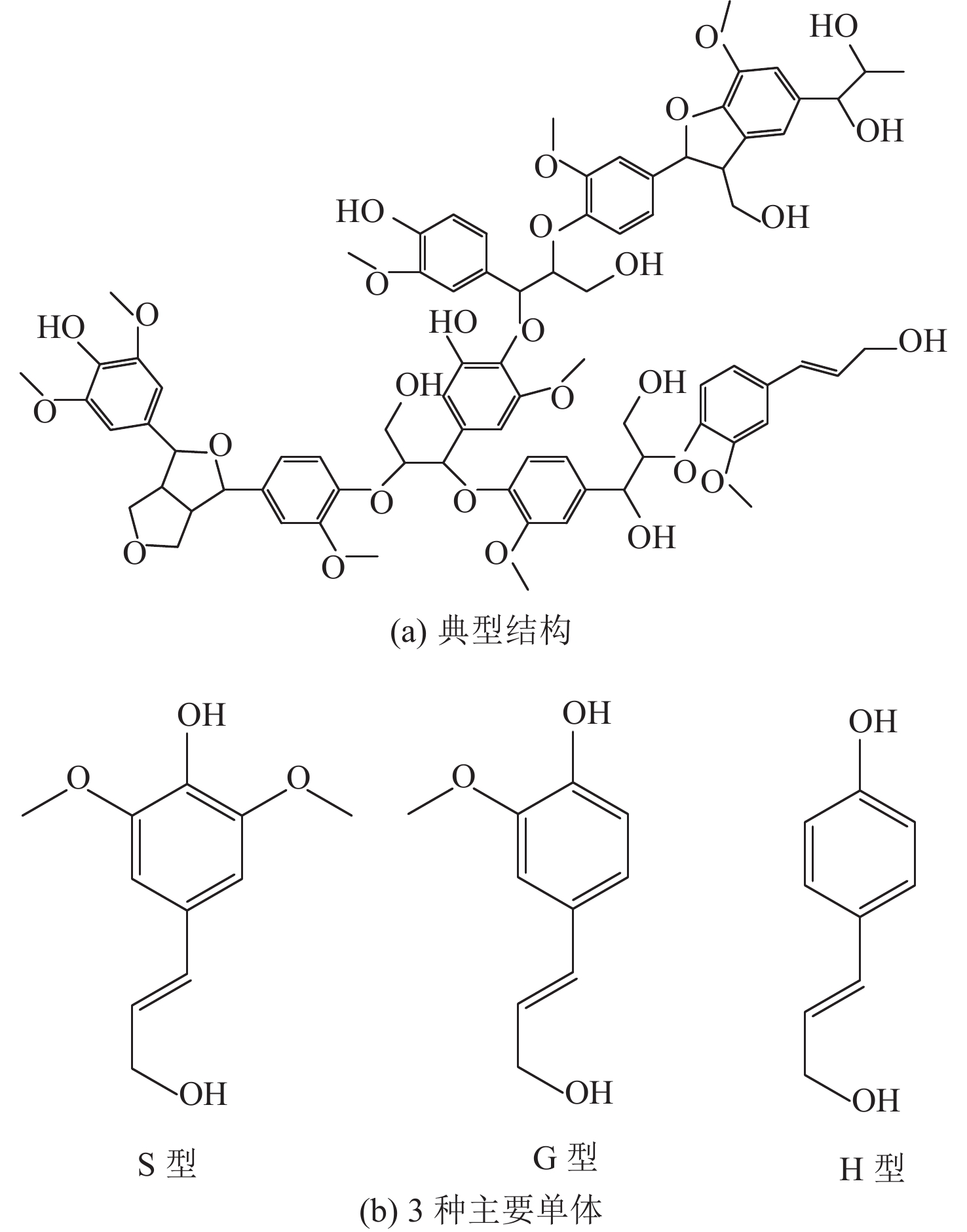
-
摘要:
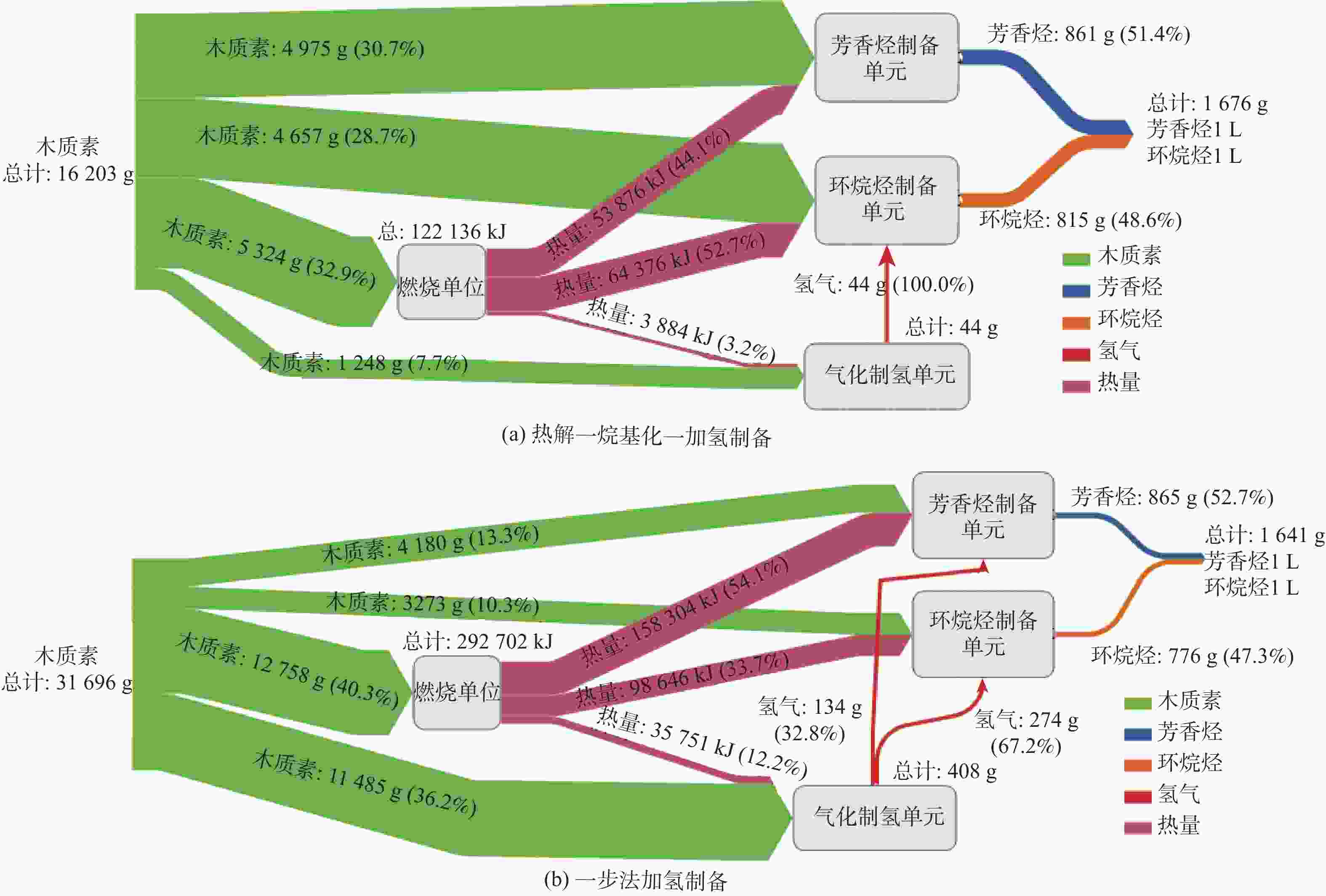
木质素单体的环状结构使其成为航空替代燃料中芳香烃和环烷烃制备的有效前驱体。通过比较分析木质素加氢制备芳香烃和环烷烃、气化制氢、燃烧供热路径的工艺特点和物流信息,采用Aspen Plus进行不同路径的物耗及能耗分析。其中木质素制备芳香烃收率为17.3%~20.7%,环烷烃收率为17.5%~23.7%,气化制氢收率为35.519 g/kg木质素,燃烧供热为22.942 MJ/kg木质素。基于充分利用木质素的原则,以木质素气化制氢作为氢源,木质素燃烧作为热源,结合芳香烃和环烷烃制备路径,设计了多条木质素制备航油自供氢自供热的综合工艺。通过能耗分析和优化,得出木质素热解-烷基化-加氢制备芳香烃或环烷烃综合工艺航油综合收率为11.8%或9.2%,综合热负荷为7.357 MJ/kg或7.687 MJ/kg木质素;木质素热解-烷基化-加氢制备芳香烃和环烷烃(1∶1)综合工艺航油综合收率为10.3%,综合热负荷为7.538 MJ/kg木质素,综合氢耗为0.27%。木质素一步法加氢制备航油工艺虽然反应条件温和,流程简单,但仍需进一步改善反应体系以解决高能耗的问题。
Abstract:The cyclic structure of lignin monomer makes it an effective precursor for the preparation of aromatic and naphthenic hydrocarbons in alternative aviation biofuel. Aspen Plus conducted a material and energy consumption analysis of various routes by comparing the features and material flow of lignin depolymerization-hydrotreating for the production of aromatic and naphthenic hydrocarbons, gasification for the production of hydrogen, and combustion for heat. The yield of aromatic hydrocarbons from lignin is 17.3%~20.7%; naphthenic hydrocarbons from lignin is 17.5%~23.7%; hydrogen from lignin is 35.519 g/kg lignin; and the heat from combustion is 22.942 MJ/kg lignin. In order to make full use of lignin, several integrated processes for biofuel production were designed with hydrogen supply from lignin gasification and heat supply from lignin combustion. By using energy analysis and optimization, the integrated process of pyrolysis-alkylation-hydrotreating for aromatic or naphthenic hydrocarbons produces a yield of 11.8% or 9.2%, with a heat load of 7.357 MJ/kg lignin or 7.687 MJ/kg lignin; for aromatic and naphthenic hydrocarbons (1∶1), the integrated process of lignin pyrolysis-alkylation-hydrotreating produces a yield of 10.3%, with a heat load of 7.538 MJ/kg lignin and hydrogen consumption of 0.27%. Although the process of lignin one-step hydrotreating for biofuel production is mild and simple, the reaction system still needs to be further improved for its significant energy consumption.
-
Key words:
- lignin /
- aviation biofuel /
- hydrotreating /
- aromatic hydrocarbons /
- naphthenic hydrocarbons /
- energy analysis
-
表 1 不同木质素气化制氢工艺的反应条件及产物对比
Table 1. Characteristics of various types of lignin gasification processes for preparation of hydrogen
表 2 常见木质素类生物质的木质素含量及热值
Table 2. Lignin content and higher heat value of common lignocellulosic biomasses
类型 生物质原料 木质素含量/% HHV/(MJ·kg−1) 软木 松树 30.0 20.24 门氏绿豆杉 33.9 19.66 大西洋雪松 34.5 20.36 硬木 桉树 25.8 17.63 橡木 22.2 18.70 樱桃树 26.4 18.26 表 3 木质素热解-烷基化-加氢制备芳香烃和环烷烃路径能耗分析
Table 3. Energy consumption analysis of aromatic and naphthenic hydrocarbons production by lignin pyrolysis-alkylation- hydrotreating pathway
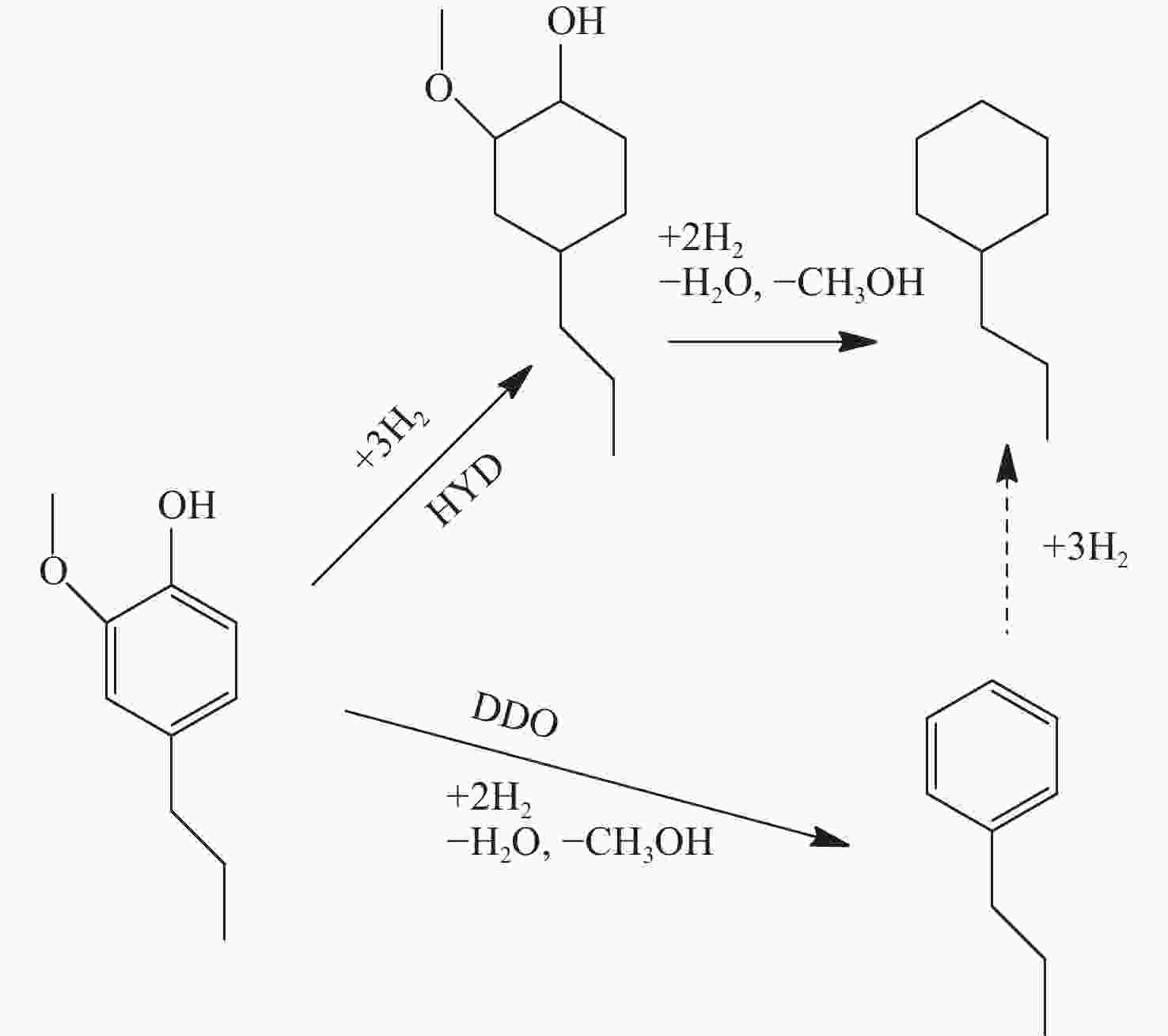
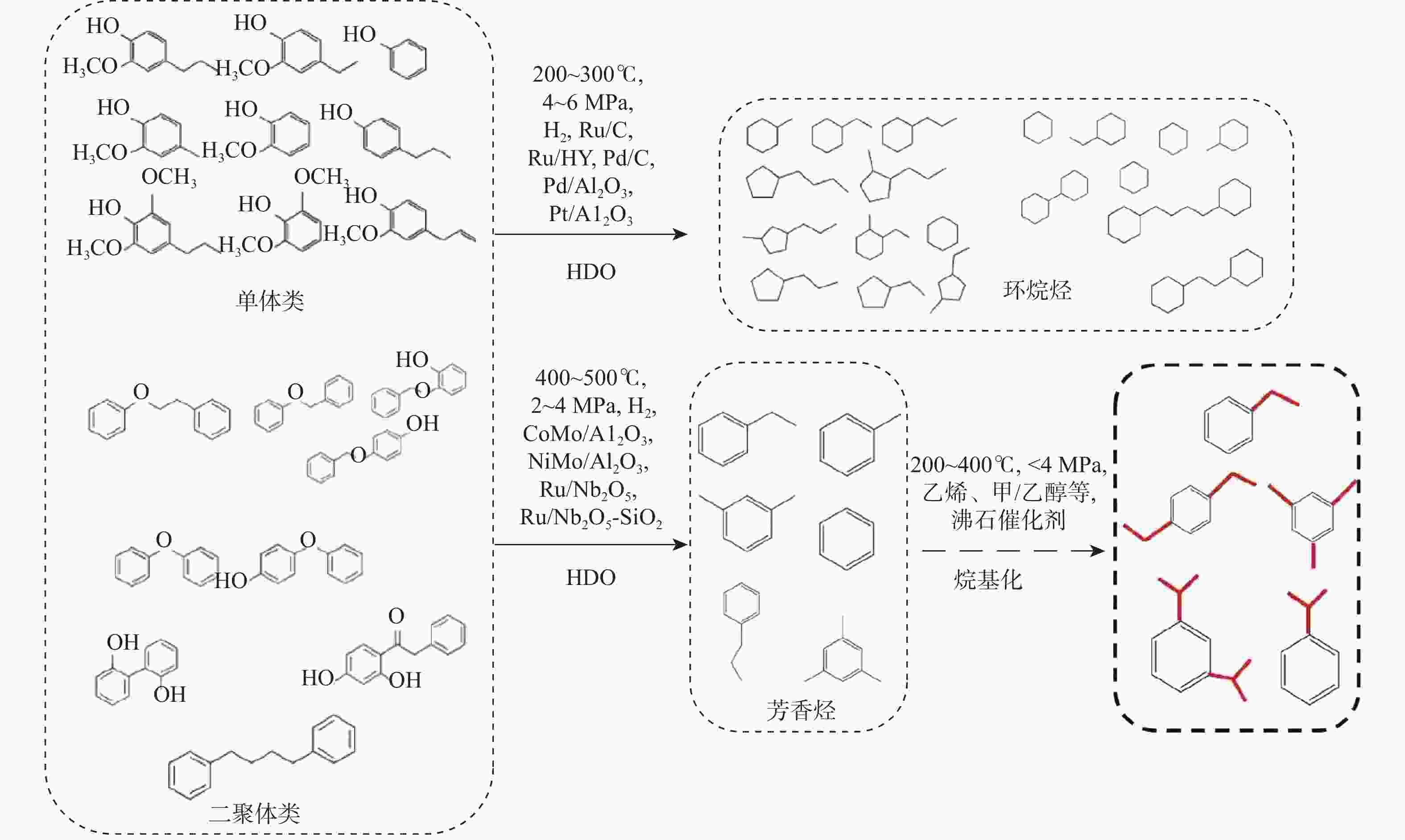
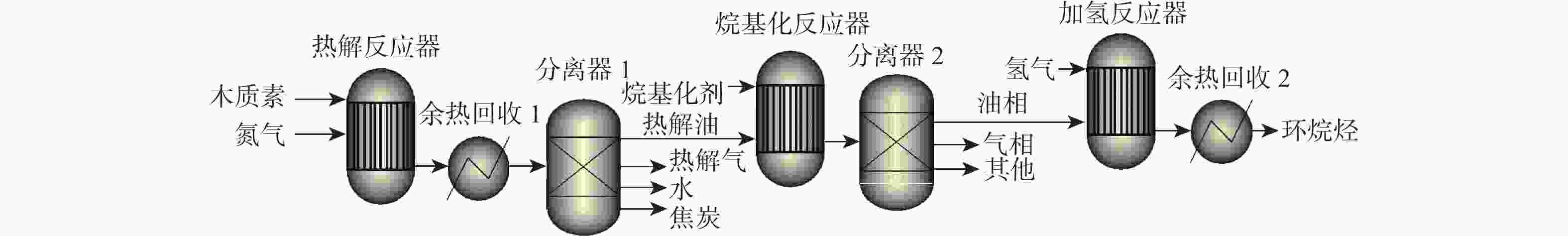
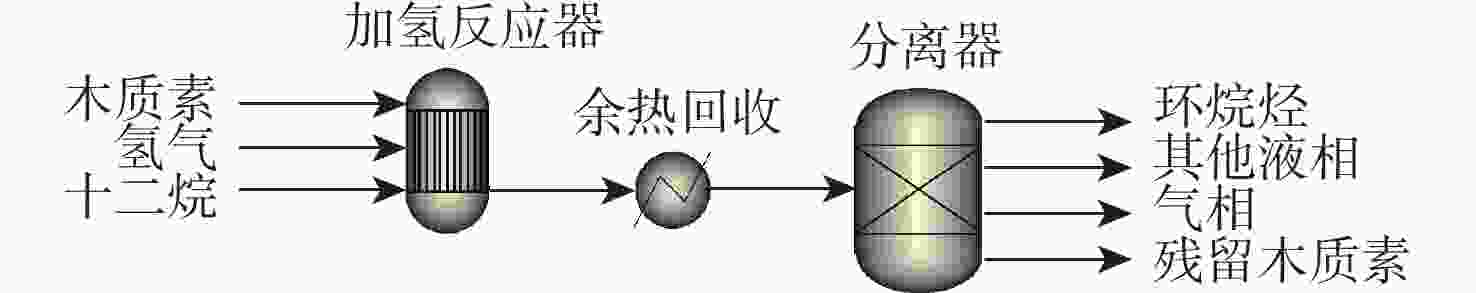
反应单元 操作条件 物质输入 物质流/kg 物质输出 物质流/kg 热负荷/(MJ·kg−1)a 热解反应器 500 ℃ 木质素 1.000 木质素 0.029 1.688 N2 0.466 N2 0.466 HZSM-5 热解油b 0.262 热解气 c 0.315 H2O 0.186 焦炭 0.208 余热回收 20 ℃、80%效率 −1.479 分离器 分离热解油 烷基化反应器 60 ℃ 热解油 b 0.262 油相:C8~C15芳香烃 0.173 10.620 烷基化剂d 0.639 油相:其他e 0.046 [bmim]Cl-2AlCl3 富余C2~C4烯烃 0.038 其他气相 0.437 其他f 0.207 分离器 分离芳香烃 加氢反应器 180 ℃、5 MPa 油相:C8~C15 芳香烃 0.219 C8~C15环烷烃 0.175 18.024 油相:其他e 0.046 其他环烷烃g 0.053 H2 0.013 富余H2 0.004 余热回收 20 ℃、80%效率 −15.029 注: a 热负荷基于每kg木质素,正值为吸热,负值为放热;b 含有 36%苯,32%甲苯,11%二甲苯,1%对乙基甲苯,2%三甲苯,1%苯酚,18%萘;c 含有 23% CO,37% CO2,35% C2~C4烷烃,5% C2~C4烯烃;d 含有 17.3% CO,35.0% CO2,7.1% C3H8,18.3% C3H6,7.5% C2H4,5.7% C4H8,2.0% CH4,7.1% C5H12;e 含有碳数6~7单环芳香烃,碳数16以上萘类碳氢化合物;f 假设为焦炭;g 含有碳数6~7单环环烷烃,碳数16以上双环环烷烃。 表 4 木质素一步法加氢制备芳香烃路径能耗分析
Table 4. Energy consumption analysis of aromatic hydrocarbons production by lignin one-step hydrotreating pathway
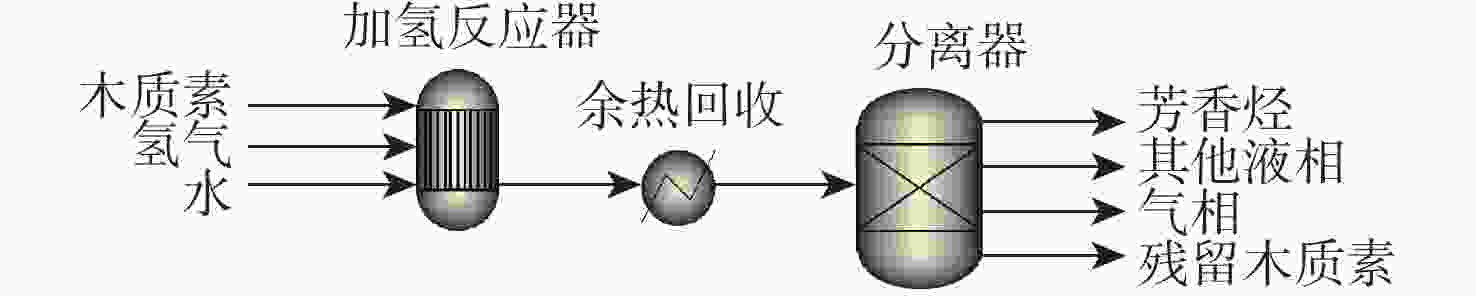
反应单元 操作条件 物质输入 物质流/kg 物质输出 物质流/kg 热负荷/(MJ·kg−1)a 加氢反应器 250 ℃、0.7 MPa H2(初始) 木质素 1.000 木质素 0.541 190.969 H2O 149.815 H2O 149.815 H2 0.287 富余H2 0.255 Ru/Nb2O5 其他气相(CH4) 0.155 芳香烃b 0.207 其他液相c 0.129 余热回收 20 ℃、80%效率 −153.102 分离器 分离芳香烃 注: a 热负荷基于每kg木质素,正值为吸热,负值为放热;b 含有13.5%甲苯,44.0%乙苯,0.5%二甲苯,41.1%丙苯,1.0%甲基乙苯;c表示环烷烃、烷烃、二苯基衍生物等。 表 5 木质素一步法加氢制备环烷烃路径能耗分析
Table 5. Energy consumption analysis of naphthenic hydrocarbons production by lignin one-step hydrotreating pathway
反应单元 操作条件 物质输入 物质流/kg 物质输出 物质流/kg 热负荷/(MJ·kg−1)a 加氢反应器 300 ℃、6 MPa H2(初始) 木质素 1.000 木质素 0.220 120.050 十二烷 29.982 十二烷 29.982 H2 0.738 富余H2 0.655 Ni/ASA 气相(98% CH4、2% C2~C4烷烃) 0.504 C8~C16环烷烃 0.237 其他液相b 0.122 余热回收 20 ℃、80%效率 −89.910 分离器 分离环烷烃 注: a 热负荷基于每kg木质素,正值为吸热,负值为放热;b 包括碳数8以下短链烷烃/环烷烃,碳数17以上长链环烷烃等。 表 6 木质素气化制备氢气路径能耗分析
Table 6. Energy consumption analysis of hydrogen production by lignin gasification pathway
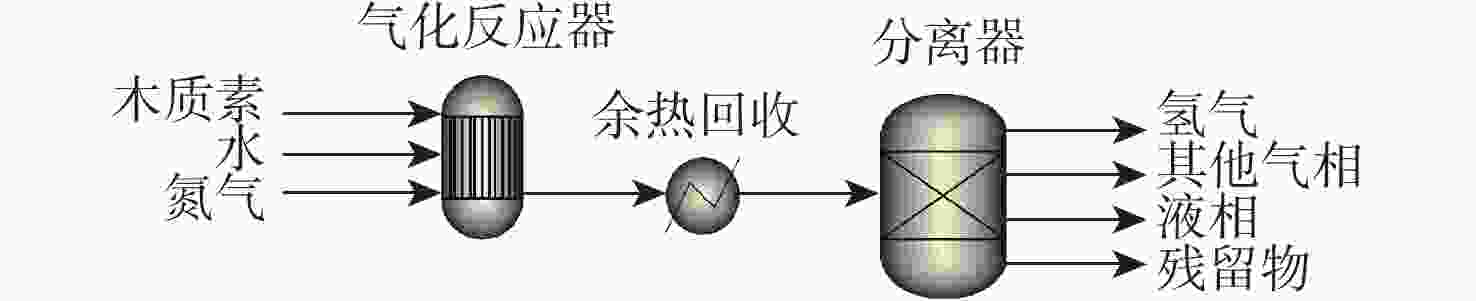
反应单元 操作条件 物质输入 物质流/kg 物质输出 物质流/kg 热负荷/(MJ·kg−1)a 气化反应器 900 ℃ 木质素 1.000 残留物b 0.480 21.483 H2O 2.400 H2O 2.369 N2 11.185 N2 11.185 Ni-Ca-Al CO 0.133 H2 0.036 CO2 0.345 CH4 0.033 C2~C4 0.004 余热回收 20 ℃、80%效率 −18.370 分离器 分离氢气 注: a 热负荷基于每kg木质素,正值为吸热,负值为放热;b 假设为未转化的木质素。 表 7 木质素燃烧供热路径能耗分析
Table 7. Energy consumption analysis of heat production by lignin combustion pathway
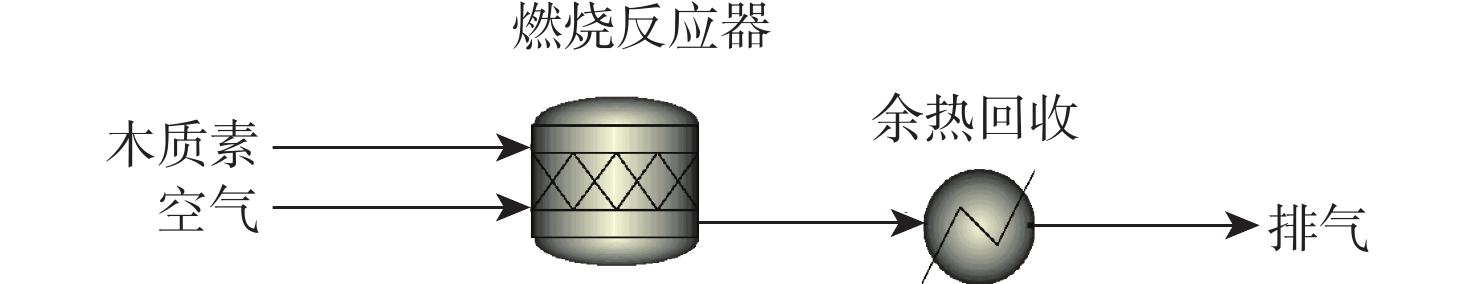
反应单元 操作条件 物质输入 物质流/kg 物质输出 物质流/kg 热负荷/(MJ·kg−1)a 燃烧反应器 1 300 ℃ 木质素 1.000 N2 6.267 −11.487 O2 1.903 H2O 0.548 N2 6.267 CO2 2.355 余热回收 20 ℃、80%效率 −11.455 注:a 热负荷基于每kg木质素,正值为吸热,负值为放热。 表 8 木质素制备航油自供氢自供热综合工艺能流分析
Table 8. Energy analysis of integrated processes of aviation biofuel production from lignin by self-supply of hydrogen and self-supply of heat
综合工艺
(自供氢自供热)航油综合
收率/% a综合热负
荷/MJ b综合
氢耗/% c木质素消耗比例/% 热量消耗比例/% 氢气消耗比例/% 芳香烃
制备单元环烷烃
制备单元制热
单元制氢
单元芳香烃
制备单元环烷烃
制备单元制氢
单元芳香烃
制备单元环烷烃
制备单元热解-烷基化
制备芳香烃11.8 7.357 67.9 32.1 100.0 热解-烷基化-加氢
制备环烷烃9.2 7.687 0.50 52.4 33.5 14.1 94.3 5.7 100.0 一步法加氢
制备芳香烃5.6 11.067 0.87 27.2 48.2 24.6 93.1 6.9 100.0 一步法加
氢制备环烷烃4.7 7.510 1.68 20.1 32.7 47.2 80.4 19.6 100.0 热解-烷基化-加氢
制备芳香烃和环烷烃(1∶1)10.3 7.538 0.27 30.7 28.7 32.9 7.7 44.1 52.7 3.2 100.0 一步法加氢制备
芳香烃和环烷烃(1∶1)5.2 9.235 1.29 13.2 10.3 40.3 36.2 54.1 33.7 12.2 32.8 67.2 注:a 航油综合收率=综合工艺航油质量/总消耗木质素质量×100%;b 综合工艺中消耗每千克(kg)木质素所需的热负荷(MJ);c综合氢耗=综合工艺氢气消耗质量/木质素质量×100%。 -
[1] 黄星华, 董升飞, 杨晓奕. 纤维素航油缩合-加氢工艺能耗分析[J]. 北京航空航天大学学报, 2022, 48(1): 121-131. doi: 10.13700/j.bh.1001-5965.2020.0506HUANG X H, DONG S F, YANG X Y. Energy consumption of condensation-hydrogenation process to prepare alkanes from lignocellulose biomass[J]. Journal of Beijing University of Aeronautics and Astronautics, 2022, 48(1): 121-131(in Chinese). doi: 10.13700/j.bh.1001-5965.2020.0506 [2] LI S, ZHENG H S, ZHENG Y J, et al. Recent advances in hydrogen production by thermo-catalytic conversion of biomass[J]. International Journal of Hydrogen Energy, 2019, 44(28): 14266-14278. doi: 10.1016/j.ijhydene.2019.03.018 [3] DIESTE A, CLAVIJO L, TORRES A I, et al. Lignin from Eucalyptus SPP. Kraft Black Liquor as Biofuel[J]. Energy & Fuels, 2016, 30(12): 10494-10498. [4] CHENG F, BREWER C E. Producing jet fuel from biomass lignin: Potential pathways to alkyl-benzenes and cycloalkanes[J]. Renewable and Sustainable Energy Reviews, 2017, 72: 673-722. doi: 10.1016/j.rser.2017.01.030 [5] AZADI P, INDERWILDI O R, FARNOOD R, et al. Liquid fuels, hydrogen and chemicals from lignin: A critical review[J]. Renewable and Sustainable Energy Reviews, 2013, 21: 506-523. doi: 10.1016/j.rser.2012.12.022 [6] CHIO C, SAIN M, QIN W S. Lignin Utilization: A review of lignin depolymerization from various aspects[J]. Renewable and Sustainable Energy Reviews, 2019, 107: 232-249. doi: 10.1016/j.rser.2019.03.008 [7] TRIBOT A, AMER G, ABDOU ALIO M, et al. Wood-lignin: Supply, extraction processes and use as bio-based material[J]. European Polymer Journal, 2019, 112: 228-240. doi: 10.1016/j.eurpolymj.2019.01.007 [8] LUO Z C, WANG Y M, HE M Y, et al. Precise oxygen scission of lignin derived aryl ethers to quantitatively produce aromatic hydrocarbons in water[J]. Green Chemistry, 2016, 18(2): 433-441. doi: 10.1039/C5GC01790D [9] HONG Y C, ZHANG H, SUN J M, et al. Synergistic catalysis between Pd and Fe in Gas phase hydrodeoxygenation of m-cresol[J]. ACS Catalysis, 2014, 4(10): 3335-3345. doi: 10.1021/cs500578g [10] GUO T Y, XIA Q N, SHAO Y, et al. Direct deoxygenation of lignin model compounds into aromatic hydrocarbons through hydrogen transfer reaction[J]. Applied Catalysis A:General, 2017, 547: 30-36. doi: 10.1016/j.apcata.2017.07.050 [11] SHAO Y, XIA Q N, DONG L, et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst[J]. Nature Communications, 2017, 8(1): 16104. doi: 10.1038/ncomms16104 [12] LASKAR D D, YANG B, WANG H M, et al. Pathways for biomass-derived lignin to hydrocarbon fuels[J]. Biofuels, Bioproducts and Biorefining, 2013, 7(5): 602-626. doi: 10.1002/bbb.1422 [13] SAIDI M, SAMIMI F, KARIMIPOURFARD D, et al. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation[J]. Energy & Environmental Science, 2014, 7(1): 103-129. [14] GUAN W X, CHEN X A, JIN S H, et al. Highly stable Nb2O5-Al2O3 composites supported pt catalysts for hydrodeoxygenation of diphenyl ether[J]. Industrial & Engineering Chemistry Research, 2017, 56(47): 14034-14042. [15] LUO Z C, ZHENG Z X, WANG Y C, et al. Hydrothermally stable Ru/hzsm-5-catalyzed selective hydrogenolysis of lignin-derived substituted phenols to bio-arenes in water[J]. Green Chemistry, 2016, 18(21): 5845-5858. doi: 10.1039/C6GC01971D [16] WANG H L, FENG M Q, YANG B. Catalytic hydrodeoxygenation of anisole: An insight into the role of metals in transalkylation reactions in bio-oil upgrading[J]. Green Chemistry, 2017, 19(7): 1668-1673. doi: 10.1039/C6GC03198F [17] WU C F, WANG Z C, DUPONT V, et al. Nickel-catalysed pyrolysis/gasification of biomass components[J]. Journal of Analytical and Applied Pyrolysis, 2013, 99: 143-148. doi: 10.1016/j.jaap.2012.10.010 [18] FERDOUS D, DALAI A K, BEJ S K, et al. Production of H2 and medium heating value gas via steam gasification of lignins in fixed-bed reactors[J]. The Canadian Journal of Chemical Engineering, 2001, 79(6): 913-922. doi: 10.1002/cjce.5450790609 [19] KANG K, AZARGOHAR R, DALAI A, et al. Hydrogen generation via supercritical water gasification of lignin using Ni-Co/Mg-Al catalysts[J]. International Journal of Energy Research, 2017, 41(13): 1835-1846. doi: 10.1002/er.3739 [20] DEMIRBAS A. Higher heating values of lignin types from wood and non-wood lignocellulosic biomasses [J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2017, 39(6): 592-598. [21] BI P Y, WANG J C, ZHANG Y J, et al. From lignin to cycloparaffins and aromatics: Directional synthesis of jet and diesel fuel range biofuels using biomass[J]. Bioresource Technology, 2015, 183: 10-17. doi: 10.1016/j.biortech.2015.02.023 [22] KONG J C, HE M Y, LERCHER J A, et al. Direct production of naphthenes and paraffins from lignin[J]. Chemical Communications, 2015, 51(99): 17580-17583. doi: 10.1039/C5CC06828B [23] SHAFER L, STRIEBICH R, GOMACH J, et al. Chemical class composition of commercial jet fuels and other specialty kerosene fuels [C]//Proceedings of the 14th AIAA/AHI Space Planes and Hypersonic Systems and Technologies Conference. Reston: AIAA, 2006. -






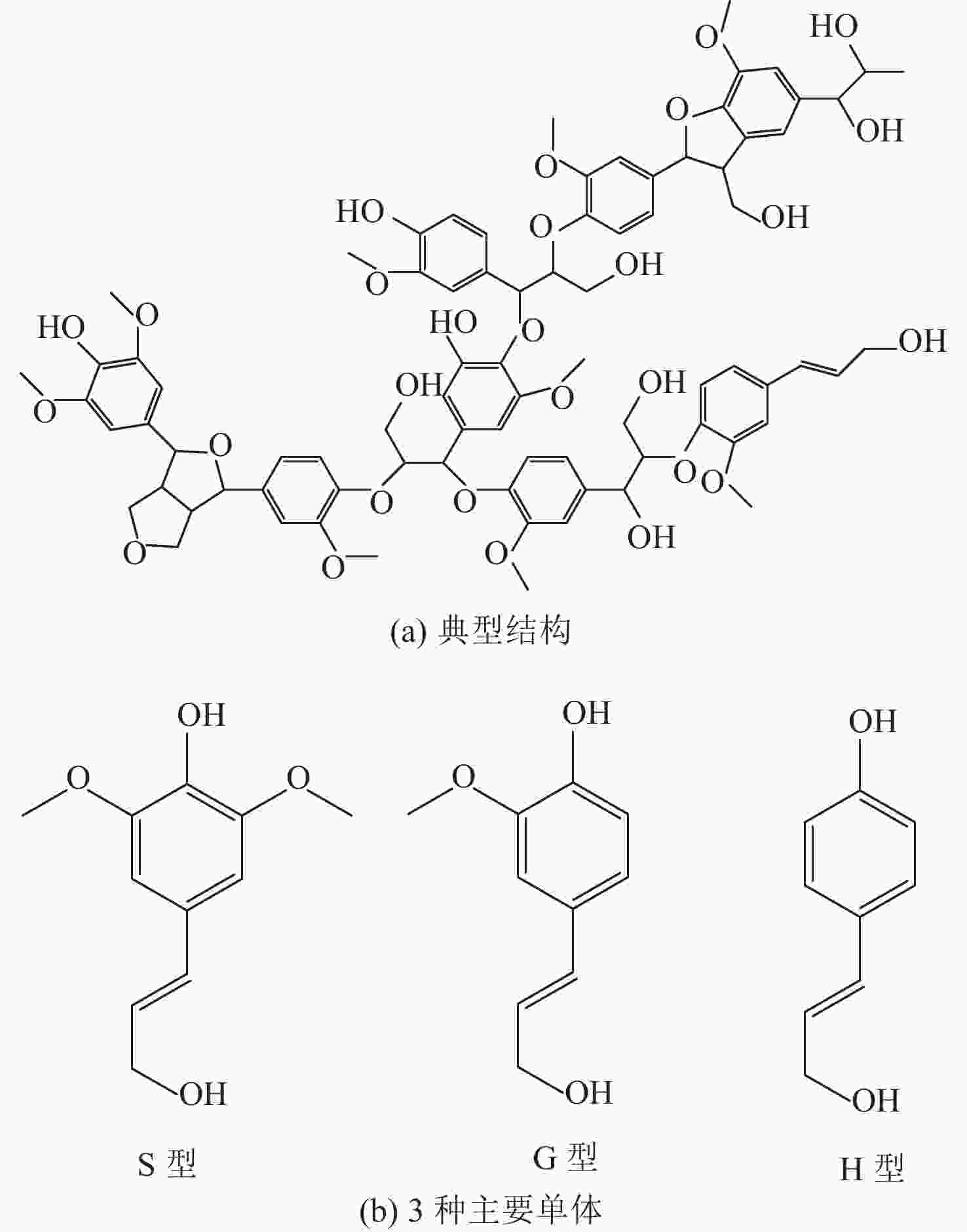
 下载:
下载: