-
摘要:
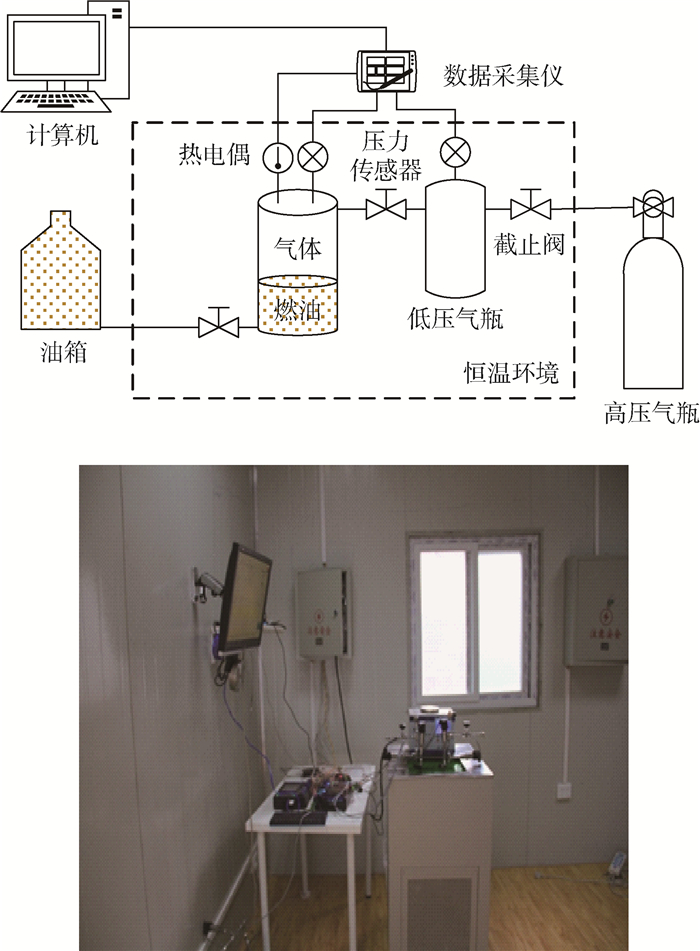
CO2在航空燃油中的质量扩散规律对飞机油箱惰化系统的研究极其重要。搭建压力降法实验装置测量CO2在RP-3航空燃油中的扩散系数,实验测试了-20、0、20、40和60℃恒温条件下的CO2气相空间压力随时间变化关系。根据Fick定律建立了容器中二维扩散方程,并采用数值解法,设定扩散系数值,求解气体在航空燃油中的浓度分布,根据质量守恒和实际气体状态方程可得到CO2气相空间压力,并与实验记录的气体压力进行比较。以扩散系数为自变量,推导了实验与理论计算误差函数,并采用Husain单一变量搜索法,使误差函数值最小,可得到扩散系数最优解。研究还显示CO2在RP-3航空燃油中的扩散系数随温度升高而增大,满足Arrhenius方程。
Abstract:The rule of mass diffusion of CO2 in jet fuel is an important consideration in the research of the aircraft fuel tank inerting system. A pressure-decay test apparatus was constructed to measure the diffusion coefficient of carbon dioxide in RP-3 jet fuel by monitoring the pressure variation at constant temperature of -20, 0, 20, 40, and 60℃, respectively. The two-dimensional diffusion equation in the hermetic container was derived based on Fick's law, and the concentration distribution was determined via the numerical method with the assumption that diffusion coefficient was known. Then pressure on ullage was predicted by utilizing mass conservation and real gas state equation and the calculating result was compared with the experimental data. By adopting the diffusion coefficient as the independent variable, the error functions as the experimental and theoretical data were derived. The optimum solution to the diffusion coefficient was obtained by Husain single variable search method to minimize the error. The study also reveals that the diffusion coefficient increases with the rise of the temperature, and the Arrhenius equation could be employed to correlate the diffusion coefficient and temperature.
-
Key words:
- carbon dioxide /
- RP-3 jet fuel /
- pressure-decay method /
- mass transfer /
- diffusion coefficient /
- optimization
-
表 1 扩散系数最优解
Table 1. Optimum solution of diffusion coefficient
T/℃ Δpave, min/kPa D/(10-8m2·s-1) -20 5.89 6.1 0 3.87 7.6 20 5.35 8.6 40 4.98 9.7 60 3.59 11.1 -
[1] JOHNSON R W, ZAKI R, YATES S F. Advanced carbon dioxide fuel tank inerting system: US 7905259B2[P]. 2011-03-15. [2] LIMAYE S, KOENIG D. Catalytic reactive component reduction system and methods for the use thereof: US 7694916B2[P]. 2010-04-13. [3] REYNOLDS T L, EKLUND T I, HAACK G A. Onboard inert gas generation system onboard oxygen gas generation system(OBIGGS/OBOGS) study: PartⅡ: Gas separation technology-state of the art: NASA/CR-2001-210950[R]. Washington, D. C. : NASA, 2001. [4] ASTM Committee. Standard test method for estimation of solubility of gases in petroleum liquids: ASTM D2780-92[S]. West Conshohocken: ASTM International, 2002. [5] 王斐, 邵晓红, 汪文川.CH4和CO2在活性炭微球内扩散系数的测定[J].化工学报, 2006, 57(8):1891-1896. http://www.whxb.pku.edu.cn/CN/abstract/abstract22647.shtmlWANG F, SHAO X H, WANG W C.Measurements of diffusion coefficients for methane and carbon dioxide in activated meso-carbon microbeads[J].Journal of Chemical Industry and Engineering, 2006, 57(8):1891-1896(in Chinese). http://www.whxb.pku.edu.cn/CN/abstract/abstract22647.shtml [6] ETMINAN S R, MAINI B B, CHEN Z X, et al.Constant-pressure technique for gas diffusivity and solubility measurements in heavy oil and bitumen[J].Energy & Fuels, 2010, 24(1):533-549. http://cat.inist.fr/?aModele=afficheN&cpsidt=22446943 [7] BEHZADFAR E, HATZIKIRIAKOS S G.Diffusivity of CO2 in bitumen:Pressure-decay measurements coupled with rheometry[J]. Energy & Fuels, 2014, 28(2):1304-1311. doi: 10.1021/ef402392r [8] ZHANG Y P, HYNDMAN C L, MAINI B B.Measurement of gas diffusivity in heavy oils[J].Journal of Petroleum Science and Engineering, 1999, 25(1-2):37-47. http://linkinghub.elsevier.com/retrieve/pii/S0920410599000315 [9] RIAZI M R, WHITSON C H.Estimating diffusion coefficients of dense fluids[J].Industrial & Engineering Chemistry Process Design & Development, 1993, 32(12):3081-3088. doi: 10.1021/ie00024a018?src=recsys [10] UNATRAKARN D, ASGHARI K, CONDOR J.Experimental studies of CO2 and CH4 diffusion coefficient in bulk oil and porous media[J].Energy Procedia, 2011, 4:2170-2177. doi: 10.1016/j.egypro.2011.02.103 [11] 严小伟, 单奕彬, 王靖岱.反相气相色谱法测定小分子溶剂在聚乙烯粒子中的无限稀释扩散系数[J].化工学报, 2007, 58(8):1917-1925. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb200708006YAN X W, SHAN Y B, WANG J D.Measurement of infinite dilution diffusion coefficients of small molecule solvents in nascent polyethylene particles by inverse gas chromatography[J].Journal of Chemical Industry and Engineering, 2007, 58(8):1917-1925(in Chinese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb200708006 [12] CIVAN F, RASMUSSEN M L. Analysis and interpretation of gas diffusion in quiescent reservoir, drilling, and completion fluids: Equilibrium vs. non-equilibrium models[C]//SPE Annual Technical Conference and Exhibition. Richardson: SPE, 2003: 1-13. [13] SHEIKHA H, DARVISH P M, MEHROTRA A K.Development of graphical methods for estimating the diffusivity coefficient of gases in bitumen from pressure-decay data[J].Energy & Fuels, 2005, 19(5):2041-2049. doi: 10.1021/ef050057c?src=recsys [14] JAMIALAHMADI M, EMADI M, STEINHAGEN H M. Diffusion coefficients of methane in liquid hydrocarbons at high pressure and temperature[J].Journal of Petroleum Science and Engineering, 2006, 53(1-2):47-60. doi: 10.1016/j.petrol.2006.01.011 [15] FARAJZADEH R, ZITHA P L J, BRUINING J. Enhanced mass transfer of CO2 into water:Experiment and modeling[J].Industrial & Engineering Chemistry Process Design & Development, 2009, 48:6423-6431. doi: 10.1021/ie801521u [16] DING C, FAN Y. Measurement of diffusion coefficients of air in silicone oil and in hydraulic oil[J].Chinese Journal of Chemical Engineering, 2011, 19(2):205-211. doi: 10.1016/S1004-9541(11)60155-9 [17] BOCHNER N, PIPMAN J.A simple method of determining diffusion constants by holographic interferometry[J].Journal of Physics D:Applied Physics, 1976, 9:1825-1830. doi: 10.1088/0022-3727/9/13/003 [18] 朱春英, 马友光, 赵长伟.氨基酸在水溶液中的扩散系数[J].化工学报, 2005, 56(2):243-245. http://d.wanfangdata.com.cn/Periodical_hgxb200502010.aspxZHU C Y, MA Y G, ZHAO C W.Diffusion coefficients of amino acids in aqueous solutions[J].Journal of Chemical Industry and Engineering, 2005, 56(2):243-245(in Chinese). http://d.wanfangdata.com.cn/Periodical_hgxb200502010.aspx [19] COLOMBANI J, BERT J.Holographic interferometry for the study of liquids[J].Journal of Molecular Liquids, 2007, 134(1-3):8-14. doi: 10.1016/j.molliq.2006.12.013 [20] GHOLAMI Y, AZIN R, FATEHI R, et al.Suggesting a numerical pressure-decay method for determining CO2 diffusion coefficient in water[J].Journal of Molecular Liquids, 2015, 211:31-39. doi: 10.1016/j.molliq.2015.06.060 [21] NGUYEN T A, ALI S M F. Role of diffusion and gravity segregation in oil recovery by the immiscible carbon dioxide wag process[C]//Conference: 6. UNITAR International Conference on Heavy Crude and Tar Sands on Fueling for a Clean and Safe Environment. New York: UNITAR, 1995: 393-403. [22] THARANIVASAN A K, YANG C D, GU Y G.Comparison of three different interface mass transfer models used in the experimental measurement of solvent diffusivity in heavy oil[J].Journal of Petroleum Science and Engineering, 2004, 44(3-4):269-282. doi: 10.1016/j.petrol.2004.03.003 [23] RONGY L, HAUGEN K B, FIROOZABADI A.Mixing from Fickian diffusion and natural convection in binary non-equilibrium fluid phases[J] AIChE Journal, 2012, 58(5):1336-1345. doi: 10.1002/aic.v58.5 [24] SCHMIDT T, LESHCHYSHYN T H, PUTTAGUNTA V R. Diffusion of carbon dioxide into Athabasca bitumen[C]//33rd Annual Technical Meeting of the Petroleum Society of CIM. Richardson: SPE, 1982: 82-100. [25] GHOLAMI Y, AZIN R, FATEHI R, et al.Prediction of carbon dioxide dissolution in bulk water under isothermal pressure decay at different boundary conditions[J].Journal of Molecular Liquids, 2015, 202:3-33. http://www.sciencedirect.com/science/article/pii/S0167732214005662 [26] HUSAIN A G K.Optimization techniques for chemical engineers[M].Noida:Macmillan of India, 1976:63-65. [27] UPRETI S R, MEHROTRA A K.Diffusivity of CO2, CH4, C2H6 and N2 in Athabasca bitumen[J].The Canadian Journal of Chemical Engineering, 2002, 80(1):116-125. doi: 10.1002/(ISSN)1939-019X [28] NAPOLEON O, UMESL R P D. Predicting diffusion coefficients in nonpolar solvents[J].Industrial & Engineering Chemistry Process Design & Development, 1981, 20(4):662-665. doi: 10.1021/i200015a014 -






 下载:
下载: