Synthesis and characterization of high purity V2AlC prepared by pressureless sintering
-
摘要:
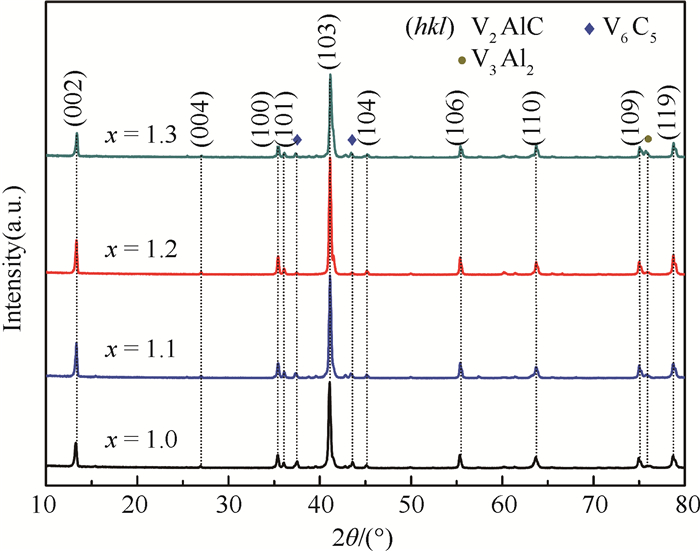
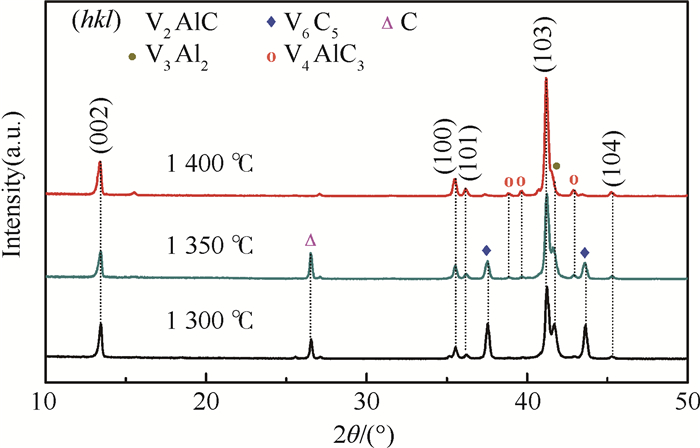
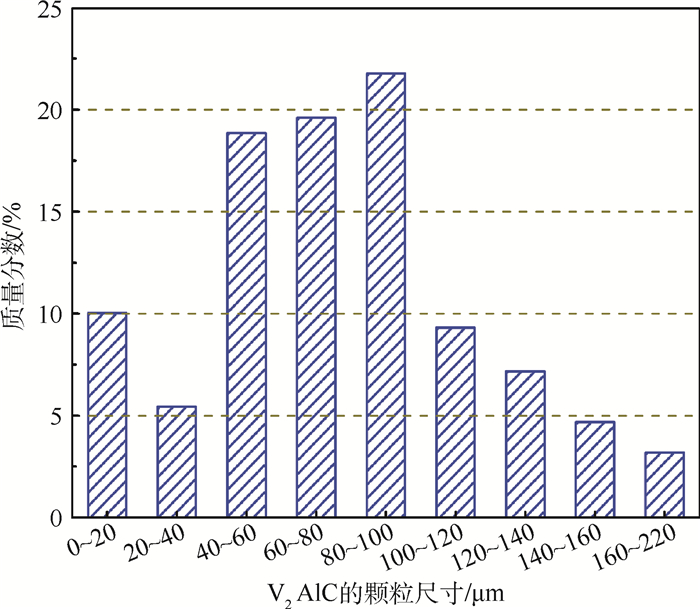
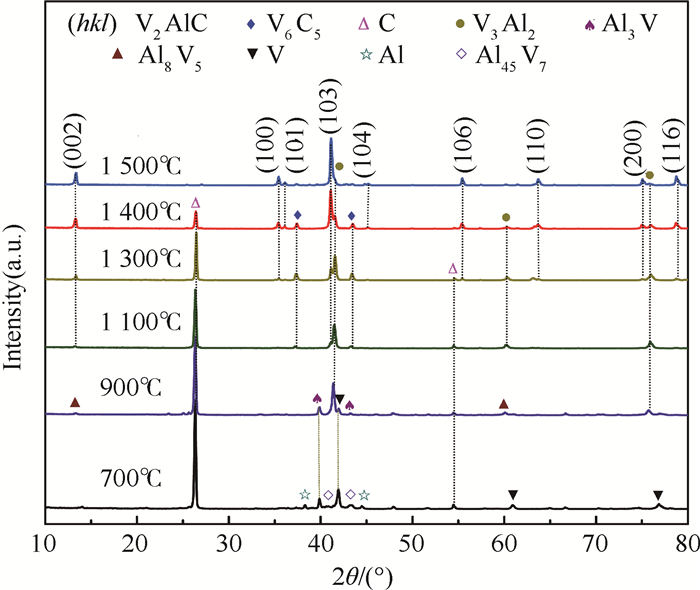
采用无压烧结的方法,以V、Al、C混合粉末为原料制备V2AlC粉体材料。通过不同烧结温度下物相的演变过程对反应路径进行研究,同时探究了烧结助剂NaF对烧结过程的影响。实验结果表明,在1 300~1 500℃温度区间内V3Al2、C和VC发生反应生成V2AlC相,且无压烧结制备高纯V2AlC的最佳工艺为1 500℃保温2 h,元素摩尔配比为V:Al:C=2:1.2:1。此外,烧结助剂NaF的使用加快了反应过程,并使得反应温度降至1 400℃。实验得到的高纯度、颗粒尺寸分布适中(40~100 μm)的V2AlC可用做提高材料耐磨性的增强体以及二维材料V2C的前驱体。
Abstract:In this paper, we reported the synthesis of high purity V2AlC by pressureless sintering with V, Al and C mixed powders. The reaction paths were studied and discussed according to the phase transformation at different sintering temperatures. Besides, the effect of sintering aid NaF on singtering process was stuied. The experimental results show that V2AlC was synthesized by the reaction of V3Al2, VC and C within the temperature range of 1 300-1 500℃ and that high purity V2AlC could be obtained with the optimized molar ratio of V:Al:C=2:1.2:1 at 1 500℃ for 2 h. In addition, the use of sintering aid NaF could greatly promote the reaction process and reduce the reaction temperature to 1 400℃. Based on the high purity and suitable size distribution (40~100 μm), the synthesized powders can be easily used as the reinforcement material or V2C precursor material.
-
Key words:
- MAX phase /
- V2AlC /
- pressureless sintering /
- reaction path /
- sintering aid
-
表 1 不同温度下的物相组成
Table 1. Phase composition at different temperatures
温度/℃ 物相组成 700 V, Al, C, Al45V7, Al3V 900 V, C, Al3V, Al8V5, V3Al2 1 100 C, V3Al2, V2AlC, V6C5 1 300 C, V3Al2, V2AlC, V6C5 1 400 V2AlC, V3Al2, V6C5, C 1 500 V2AlC, V3Al2 -
[1] WANG J Y, ZHOU Y C.Recent progress in theoretical prediction, preparation, and characterization of layered ternary transition-metal carbides[J].Annual Review of Materials Research, 2009, 39(39):415-443. [2] BARSOUM M W.The MN+1AXN phases:A new class of solids:T hermodynamically stable nanolaminates[J].Progress in Solid State Chemistry, 2000, 28(1):201-281. [3] SUN Z M, MUSIC D, AHUJA R, et al.Bonding and classification of nanolayered ternary carbides[J].Physical Review B, 2004, 70(9):092102. doi: 10.1103/PhysRevB.70.092102 [4] DAHLQVIST M, ALLING B R, ROS N J.Stability trends of MAX phases from first principles[J].Physical Review B, 2010, 81(22):220102. doi: 10.1103/PhysRevB.81.220102 [5] SUN Z M.Progress in research and development on MAX phases:A family of layered ternary compounds[J].International Materials Reviews, 2011, 56(3):143-166. doi: 10.1179/1743280410Y.0000000001 [6] GUPTA S, BARSOUM M W.Synthesis and oxidation of V2AlC and (Ti0.5, V0.5)2AlC in air[J].Journal of the Electrochemical Society, 2004, 151(2):D24-D29. doi: 10.1149/1.1639160 [7] HETTINGER J, LOFLAND S, FINKEL P, et al.Electrical trans-port, thermal transport, and elastic properties of M2AlC (M=Ti, Cr, Nb, and V)[J].Physical Review B, 2005, 72(11):115-120. [8] HU C F, HE L F, LIU M Y, et al.In situ reaction synthesis and mechanical properties of V2AlC[J].Journal of the American Ceramic Society, 2008, 91(12):4029-4035. doi: 10.1111/jace.2008.91.issue-12 [9] NAGUIB M, MOCHALIN V N, BARSOUM M W, et al.25th anniversary article:MXenes:A new family of two-dimensional materials[J].Advanced Materials, 2014, 26(7):992-1005. doi: 10.1002/adma.201304138 [10] CHEN J, CHEN K, TONG D Y, et al.CO2 and temperature dual responsive "Smart" MXene phases[J].Chemical Communications, 2015, 51(2):314-317. doi: 10.1039/C4CC07220K [11] WANG L, YUAN L Y, CHEN K, et al.Loading actinides in multi-layered structures for nuclear waste treatment:The first case study of uranium capture with vanadium carbide MXene[J].ACS Applied Materials & Interfaces, 2016, 8(25):16396-16403. [12] CHEN J, XIAO P, GU J C, et al.A smart hybrid system of Au nanoparticle immobilized PDMAEMA brushes for thermally adjustable catalysis[J].Chemical Communications, 2014, 50(10):1212-1214. doi: 10.1039/C3CC47386D [13] SHI L, OUISSE T, SARIGIANNIDOU E, et al.Synthesis of single crystals of V2AlC phase by high-temperature solution growth and slow cooling technique[J].Acta Materialia, 2015, 83:304-309. doi: 10.1016/j.actamat.2014.10.018 [14] LI X, LI X, LIANG B Y.Synthesis of phase purity V2AlC via self-propagation high temperature sintering[J].International Journal of Materials Research, 2013, 104(6):603-605. doi: 10.3139/146.110929 [15] SUN Z M, ZHANG Y, ZHOU Y C.Synthesis of Ti3SiC2 powders by a solid-liquid reaction process[J].Scripta Materialia, 1999, 41(1):61-66. doi: 10.1016/S1359-6462(99)00054-8 [16] HALLSTEDT B.Thermodynamic evaluation of the Al-V-C system[J].Calphad-Computer Coupling of Phase Diagrams and Thermochemistry, 2013, 41(6):156-159. [17] LIPATNIKOV V, GUSEV A, ETTMAYER P, et al.Phase transf-ormations in non-stoichiometric vanadium carbide[J].Journal of Physics:Condensed Matter, 1999, 11:163-184. doi: 10.1088/0953-8984/11/1/014 [18] HU C F, ZHANG J, BAO Y W, et al.In-situ reaction synthesis and decomposition of Ta2AlC[J].International Journal of Materials Research, 2008, 99(1):8-13. doi: 10.3139/146.101598 [19] PERDEW J P, BURKE K, ERNZERHOF M.Generalized gradient approximation made simple[J].Physical Review Letters, 1996, 77(18):3865-3868. doi: 10.1103/PhysRevLett.77.3865 [20] HU C F, ZHANG J, WANG J M, et al.Crystal structure of V4AlC3:A new layered ternary carbide[J].Journal of the American Ceramic Society, 2008, 91(2):636-639. doi: 10.1111/jace.2008.91.issue-2 -







 下载:
下载: