-
摘要:
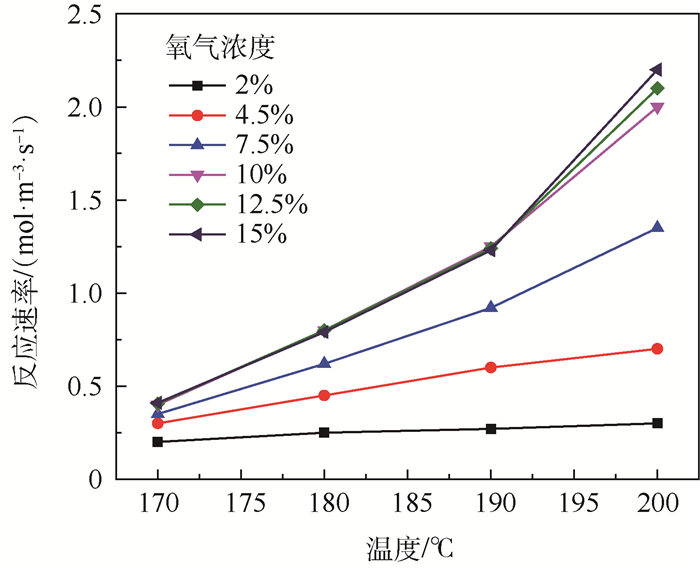
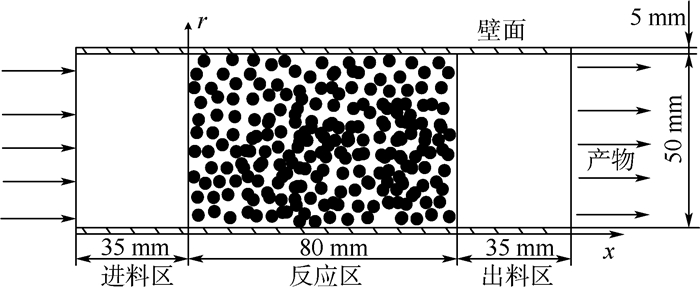
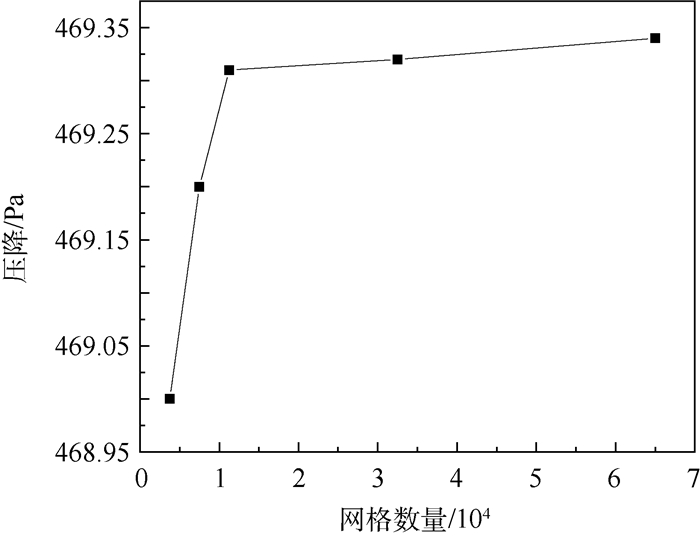
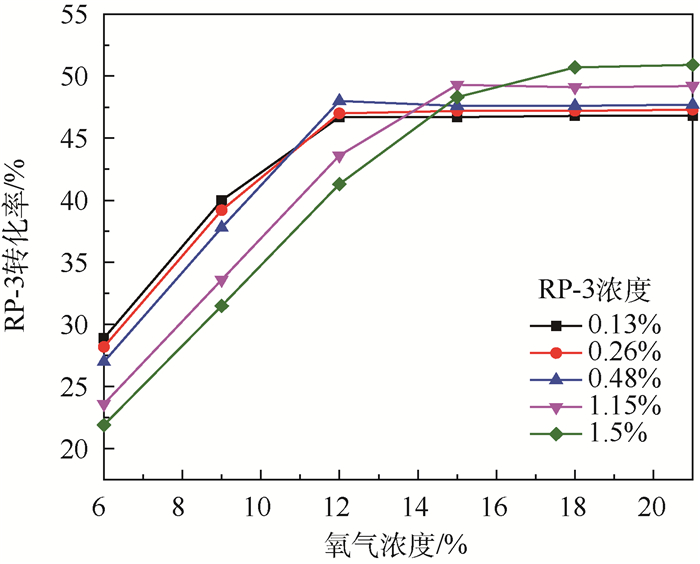
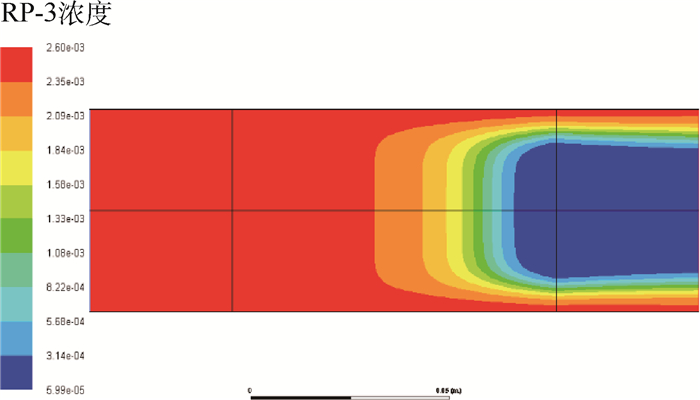
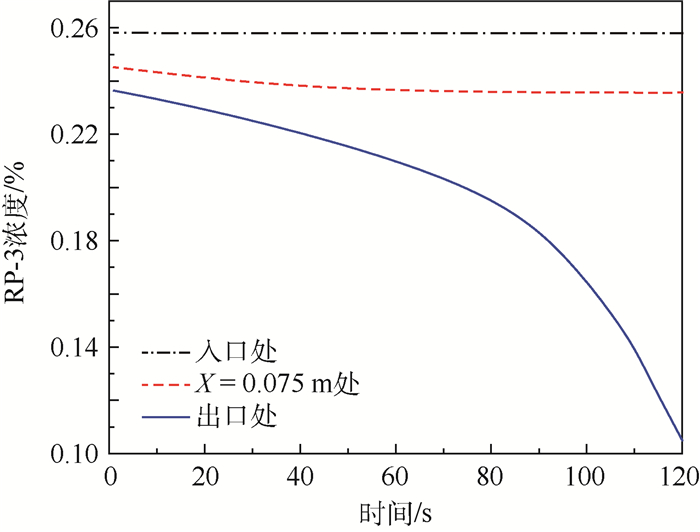
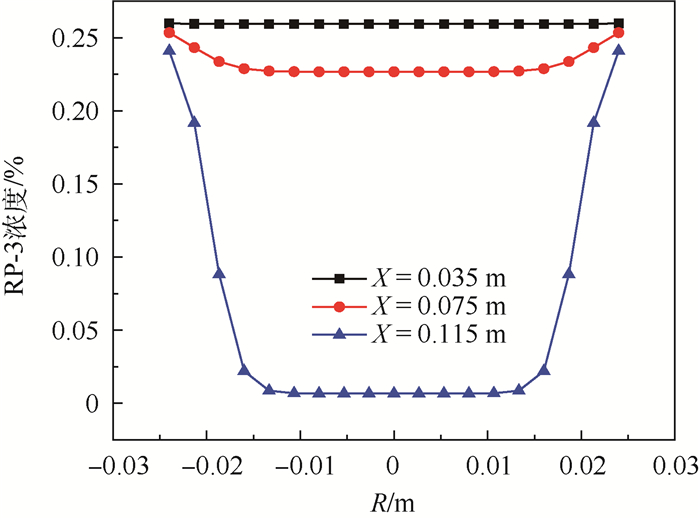
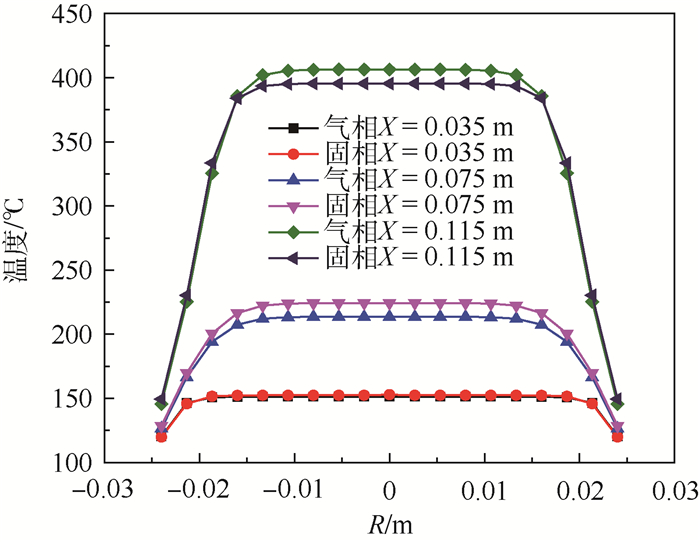
为了研究耗氧型燃油惰化系统中反应器的工作特性,在Fluent 17.0软件多孔介质模型基础上以用户自定义变量(UDS)形式添加固相能量方程来建立气-固两相耦合传热的两温度反应器模型,以大庆RP-3燃油为对象并通过实验测试了其反应动力学方程,然后以用户自定义函数(UDF)源项的形式添加化学反应,对反应器进行了仿真。研究了不同工况对反应器惰化效率的影响,以及反应器在惰化过程中的内部温度及RP-3浓度变化特性。结果显示:反应物浓度对转化率的影响与氧浓度饱和值有关系,在没有额外冷却的情况下反应器会飞温,化学反应主要发生在反应器的后半段,且靠近反应器轴线处。因此在未来设计反应器时,应当考虑额外冷却措施以防止飞温,使催化床温度均匀分布来提高反应器工作效率。
Abstract:In order to research the working performance of the reactor in oxygen consumption based fuel tank inerting system, the solid phase energy equation was added in the form of UDS to establish the two temperatures reactor model with gas-solid two-phase coupled heat transfer on the basis of Fluent 17.0 software porous medium model, the reaction kinetic equation was tested experimentally with Daqing RP-3 fuel as the object, and the chemical reaction was added in the form of UDF source terms to simulate the reactor. This paper studied the effects of different operating conditions on the inerting efficiency of the reactor, as well as the internal temperature of the reactor in inerting process and variation characteristics of RP-3 concentration. The results show that the effect of reactant concentration on conversion is related to the saturation value of oxygen concentration; the absence of additional cooling will lead to temperature run-away, and the chemical reaction mainly occurs in the second half section of the reactor and is close to the reactor axis. Therefore, when designing the reactor in the future, additional cooling measures should be considered to prevent the temperature run-away and make the temperature of the catalytic bed evenly distributed to improve the work efficiency of the reactor.
-
Key words:
- catalytic combustion /
- reactor /
- fuel tank /
- inerting system /
- RP-3
-
表 1 反应器催化床主要参数
Table 1. Main parameters of reactor catalytic bed
参数 数值 比热容/(J·kg-1·K-1) 600 密度/(kg·m-3) 2800 导热系数/(W·m-1·K-1) 0.16 直径/mm 3.5 孔隙率 0.38 -
[1] 刘卫华, 冯诗愚.飞机燃油箱惰化技术[M].北京:科学出版社, 2018:2-3.LIU W H, FENG S Y.Aircraft fuel tank inerting technology[J]. Beijing:Science Press, 2018:2-3(in Chinese). [2] ALANKAR G.Method and system for making a fuel-tank inert without an inert gas[J]. SAE International Journal of Aerospace, 2009, 2(1):75-82. doi: 10.4271/2009-01-3134 [3] VANSANT E F.Gas separation technology[M]. Amsterdam: Elsevier, 1990. [4] 王明波, 邵垒, 潘俊, 等.耗氧型燃油箱惰化技术历史和现状[J].航空科学技术, 2016, 27(7):1-7.WANG M B, SHAO L, PAN J, et al.History and current status of oxygen consumption based fuel tank inerting technology[J]. Aeronautical Science & Technology, 2016, 27(7):1-7(in Chinese). [5] JOHNSON R W, ZAKI R, YATES S F.Advanced carbon dioxide fuel tank inerting system: US2008128048A1[P]. 2008-06-05. [6] LIMAYE S, KOENIG D.Catalytic reactive component reduction system and methods for the use thereof: US2008019376 A1[P]. 2008-08-21. [7] LIMAYE S Y, ROBERTSON S, KOENIG D, et al.Reactive component reduction system and methods for the use thereof: US7896292B2[P]. 2011-03-01. [8] ROBERT J R, MORRIS W, MILLER J, et al.Fuel de-oxygenation and aircraft thermal management: AIAA-2006-4027[R]. Reston, VA: AIAA, 2006. [9] STUART R.WESLEY J, DONALD K, et al.Development of green on-board inert gas generation system (GOBIGGSTM)[EB/OL]. (2007-03-21)[2019-01-17]. [10] STUART R.Announces successful FAA testing of its fuel tank safety system, to prevent TWA 800 type explosions[EB/OL]. (2007-05-15)[2019-01-17]. [11] CHENG S H, CHENG Z C, NAN C Y, et al.Research progress in catalytic combustion of volatile organic compounds[J]. Modern Chemical Industry, 2015, 35(6):57-61. [12] 卢泽湘, 范立维.金属基整体式催化剂的研究进展[J].广州化工, 2010, 38(6):9-12. doi: 10.3969/j.issn.1001-9677.2010.06.004LU Z X, FAN L W.Research progress of metal-based monolithic catalysts[J]. Guangzhou Chemical Industry, 2010, 38(6):9-12(in Chinese). doi: 10.3969/j.issn.1001-9677.2010.06.004 [13] BENEDETTOA A D, LANDI G, SARLI V D, et al.Methane catalytic combustion under pressure[J]. Catalyst Today, 2012, 197:206-213. doi: 10.1016/j.cattod.2012.08.032 [14] SMITH R J B, MURUGANANDAM L, SHEKHAR S M.CFD analysis of low temperature water gas shift reactor[J]. Computer & Chemical Engineering, 2011, 35(12):2646-2652. [15] GAO X, ZHU Y P, LUO Z H, et al.CFD modeling of gas flow in porous medium and catalytic coupling reaction from carbon monoxide to diethyl oxalate in fixed-bed reactors[J]. Chemical Engineering Science, 2011, 66(23):6028-6038. doi: 10.1016/j.ces.2011.08.031 [16] GUARDO A, COUSSIRAT M, LARRAYOZ M, et al.CFD flow and heat transfer in nonregular packings for fixed bed equipment design[J]. Industrial & Engineering Chemistry, 2004, 43(22):7049-7056. -








 下载:
下载: