-
摘要:
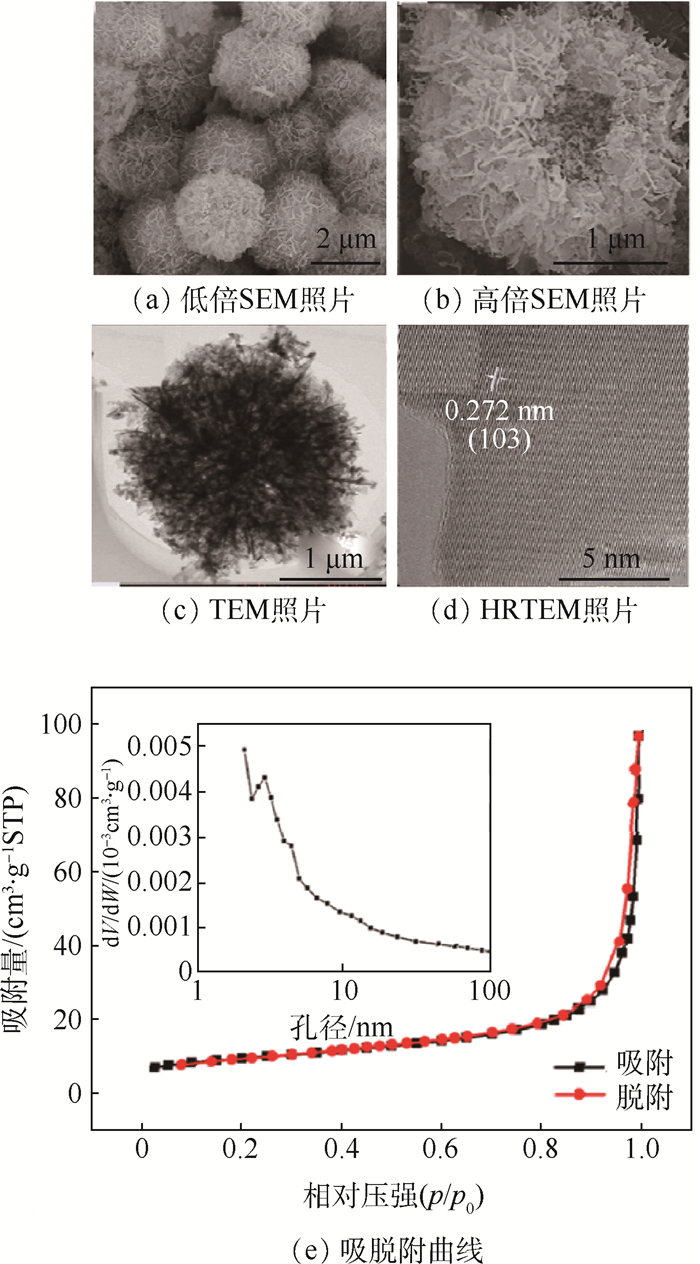
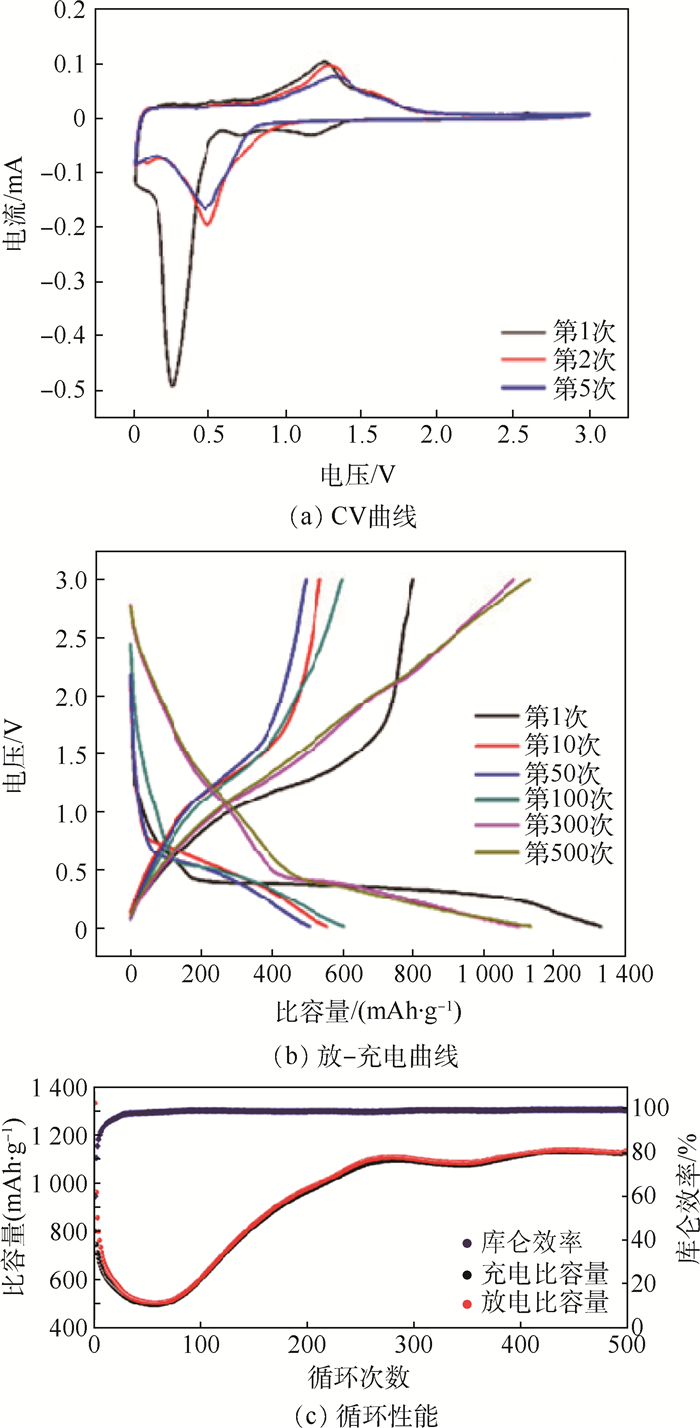
利用水热法合成了Zn-Mn氧化物前驱体,在温度400、500、600、700℃下,空气气氛中煅烧前驱体,以此来制备纳米片组装成的分级多孔结构的ZnMn2O4微球。其中,在500℃空气中煅烧前驱体制备的ZnMn2O4(ZMO-500)微球具有丰富的多级次孔结构,其作为锂离子电池负极材料,在500 mA/g的电流密度下,ZMO-500微球负极材料循环500次以后仍具有1 132 mAh/g高的放电比容量。ZMO-500负极材料优异的电化学性能得益于其分级多孔结构,不仅可以增加电极和电解质之间的接触面积以促进锂离子的迁移,而且还为循环过程中电极体积膨胀提供足够的缓冲空间。
Abstract:The precursor of Zn-Mn oxides were synthesized by a facile hydrothermal method and subsequently calcined at different temperature of 400℃, 500℃, 600℃ and 700℃ in air in order to synthesis the hierarchical porous ZnMn2O4 microspheres assembled by a lot of nanosheets. The ZnMn2O4 microspheres synthesized by calcining precursor in air at 500℃ (ZMO-500) display rich hierarchical porous structures, and when used as the anode material of lithium ion batteries, ZMO-500 microsphere anode material exhibits a high discharge capacity of 1 132 mAh/g after 500 cycles at a current density of 500 mA/g. It is believed that the outstanding electrochemical performance of ZMO-500 microsphere anode material benefits from the hierarchical porous structure that can not only increase the contact area between the electrode and the electrolyte to facilitate the transfer of Li+, but also provide sufficient space for volume expansion of the electrode during the cyclic process.
-
Key words:
- hydrothermal synthesis /
- ZnMn2O4 /
- hierarchical porous microsphere /
- anode /
- lithium ion batteries
-
表 1 不同循环次数ZMO-500电极的Warburg阻抗系数和锂离子扩散系数
Table 1. Warburg impedance coefficient and lithium ion diffusion coefficient of ZMO-500 electrodes at different cycles
循环次数 σ/(Ω·cm2·s-0.5) D/(cm2·s-1) 第1次 36.17 1.70×10-14 第50次 66.66 4.99×10-15 第200次 36.37 1.68×10-14 -
[1] DUNN B, KAMATH H, TARASCON J M.Electrical energy storage for the grid:A battery of choices[J].Science, 2011, 334(6058):928-935. doi: 10.1126/science.1212741 [2] DENG Y, WAN L, XIE Y, et al.Recent advances in Mn-based oxides as anode materials for lithium ion batteries[J].RSC Advances, 2014, 4(45):23914-23935. doi: 10.1039/C4RA02686A [3] GOODENOUGH J B, KIM Y.Challenges for rechargeable Li batteries[J].Chemistry of Materials, 2010, 22:587-603. doi: 10.1021/cm901452z [4] ETACHERI V, MAROM R, ELAZARI R, et al.Challenges in the development of advanced Li-ion batteries:A review[J].Energy & Environmental Science, 2011, 4:3243-3262. [5] PATIL A, PATIL V, WOOK SHIM D, et al.Issue and challenges facing rechargeable thin film lithium batteries[J].Materials Research Bulletin, 2008, 43:1913-1942. doi: 10.1016/j.materresbull.2007.08.031 [6] COURTEL F M, DUNCAN H, ABU-LEBDEH Y, et al.High capacity anode materials for Li-ion batteries based on spinel metal oxides AMn2O4 (A=Co, Ni, and Zn)[J].Journal of Materials Chemistry, 2011, 21:10206-10218. doi: 10.1039/c0jm04465b [7] ZHAO Y, LI X, YAN B, et al.Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries[J].Advanced Energy Materials, 2016, 6(8):1502175. doi: 10.1002/aenm.201502175 [8] ZHANG J, YU A, Nanostructured transition metal oxides as advanced anodes for lithium-ion batteries[J].Science Bulletin, 2015, 60(9):823-838. doi: 10.1007/s11434-015-0771-6 [9] GUO N, WEI X Q, DENG X L, et al.Synthesis and property of spinel porous ZnMn2O4 microspheres[J].Applied Surface Science, 2015, 356(30):1127-1134. [10] ZHANG T, GAO Y, YUE H H, et al.Convenient and high-yielding strategy for preparing nano-ZnMn2O4 as anode material in lithium-ion batteries[J].Electrochimica Acta, 2016, 198(20):84-90. [11] ZHOU L, WU H B, ZHU T, et al.Facile preparation of ZnMn2O4 hollow microspheres as high-capacity anodes for lithium-ion batteries[J].Journal of Materials Chemistry, 2012, 22:827-829. doi: 10.1039/C1JM15054E [12] FAN B, HU A, CHEN X S, et al.Hierarchical porous ZnMn2O4 microspheres as a high-performance anode for lithium-ion batteries[J].Electrochimica Acta, 2016, 213(20):37-45. [13] ALFARUQI M H, RAI A K, MATHEW V, et al.Pyro-synthesis of nanostructured spinel ZnMn2O4/C as negative electrode for rechargeable lithium-ion batteries[J].Electrochimica Acta, 2015, 151(1):558-564. [14] BAI Z, FAN N, SUN C, et al.Facile synthesis of loaf-like ZnMn2O4 nanorods and their excellent performance in Li-ion batteries[J].Nanoscale, 2013, 5(6):2442-2447. doi: 10.1039/c3nr33211j [15] LIU Y, BAI J, MA X, et al.Formation of quasi-mesocrystal ZnMn2O4 twin-microspheres via an oriented-attachment for lithium-ion batteries[J].Journal of Materials Chemistry A, 2014, 2:14236. doi: 10.1039/C4TA02950J [16] WANG N, MA X, XU H, et al.Porous ZnMn2O4 microspheres as a promising anode material for advanced lithium-ion batteries[J].Nano Energy, 2014, 6:193-199. doi: 10.1016/j.nanoen.2014.04.001 [17] LI P, LIU J, LIU Y, et al.Three-dimensional ZnMn2O4/porous carbon framework from petroleum asphalt for high performance lithium-ion battery[J].Electrochimica Acta, 2015, 180(20):164-172. [18] ZHANG G, YU L, WU H B, et al.Formation of ZnMn2O4 ball-in-ball hollow microspheres as a high-performance anode for lithium-ion batteries[J].Advanced Materials, 2012, 24(34):4609-4613. doi: 10.1002/adma.201201779 [19] CAI D, WANG D, HUANG H, et al.Rational synthesis of ZnMn2O4 porous spheres and graphene nanocomposite with enhanced performance for lithium-ion batteries[J].Journal of Materials Chemistry A, 2015, 3:11430-11436. doi: 10.1039/C5TA00539F [20] CHEN X, ZHANG Y, LIN H, et al.Porous ZnMn2O4 nanospheres:Facile synthesis through microemulsion method and excellent performance as anode of lithium ion battery[J].Journal of Power Sources, 2016, 312(20):137-145. doi: 10.1021/nn9012675 [21] LIU X, ZHANG S, XING Y, et al.MOF-derived, N-doped porous carbon coated graphene sheets as high-performance anodes for lithium-ion batteries[J].New Journal of Chemistry, 2016, 40(11):9679-9683. doi: 10.1039/C6NJ01896C [22] SUN H, XIN G, HU T, et al.High-rate lithiation-induced reactivation of mesoporous hollow spheres for long-lived lithium-ion batteries[J].Nature Communication, 2014, 31(5):4526-4529. -








 下载:
下载: