Energy consumption for production of jet fuel precursors from cellulosic biomass by hydrothermal method
-
摘要:
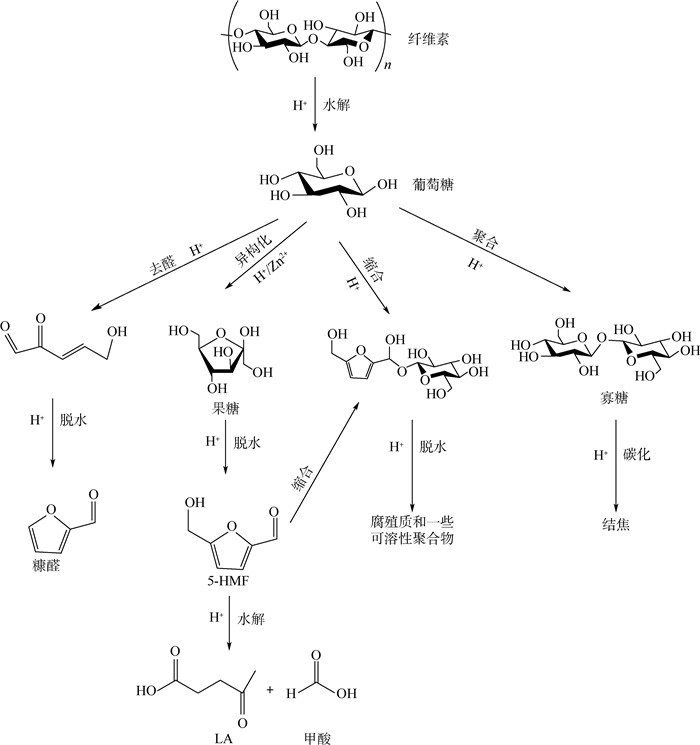
生物质制备航空替代燃料对于全球碳减排和控制温室气体排放发挥十分重要的影响。纤维素类生物质来源广、年产量大成为其作为生物质原料的显著优势。结合目前纤维素类生物质研究的最新成果,深入研究了纤维素类生物质制备航油前驱物(糠醛(FF)、5-羟甲基糠醛(5-HMF)、乙酰丙酸(LA))关键工艺单元的工艺参数和产率,通过Aspen Plus工艺模拟,研究比较了糠醛与乙酰丙酸制备工艺、糠醛与5-羟甲基糠醛制备工艺过程的物质流和能流,得出了不同工艺参数对产率的影响,并进行了能耗分析,为提高平台化合物的产率和降低能耗提供了理论基础。
Abstract:Biomass for production of alternative jet fuel has a very important impact on global carbon reduction and the control of greenhouse gas emissions. Wide sources and large annual output of cellulosic biomass have become its significant advantages as a biomass raw material to produce aviation alternative fuels. Based on the latest research results of cellulosic biomass, the process parameters and yield of the key process units for the production of jet fuel precursor (furfural (FF), 5-hydroxymethylfurfural (5-HMF) and levulinic acid (LA)) from cellulosic biomass were studied in depth in this paper. Through Aspen Plus process simulation, the material flow and energy flow of FF and LA, FF and 5-HMF were studied and compared. The influence of different process parameters on the yield was obtained, and energy consumption analysis of the advantageous process flow was carried out, which provides a theoretical basis for increasing the yield of platform compounds and reducing energy consumption of production of alternative jet fuel from biomass.
-
Key words:
- cellulosic biomass /
- alternative jet fuel /
- hydrolysis /
- energy consumption analysis /
- platform compound
-
原料 成分含量/% 纤维素 半纤维素 木质素 大麦秸秆 48.6 29.7 21.7 亚麻杆 36.7 34.4 28.9 大豆秸秆 29.2 35.5 35.3 燕麦杆 44.8 33.4 21.8 油菜秸秆 54.8 23.2 22.0 油菜杆 59.4 19.2 21.4 油菜籽壳 55.9 20.0 24.1 水稻秸秆 52.3 32.8 14.9 黑麦秸秆 49.9 29.6 20.5 小黑麦秸秆 47.9 31.5 20.6 小麦秸杆 44.5 33.2 22.3 玉米秸秆 49.0 37.9 13.1 棉花秸秆 66.2 18.4 15.4 向日葵秸杆 71.6 12.3 16.1 烟草秸秆 44.6 30.2 25.2 注:干基、无灰,标准化为100%。 表 2 纤维素、半纤维素的产率
Table 2. Yield of cellulose and hemicellulose
表 3 半纤维素水解为木糖的产率
Table 3. Yield of hemicellulose hydrolyzed to xylose
表 4 木糖转化糠醛的产率
Table 4. Yield of xylose converted to furfural
液态环境 催化剂 反应条件 产率/% 质量产率/(g·g-1) 参考文献 水/GVL(1:9) PSTA-POM 170℃,10 min 80.4 0.515 [23] 水/GVL(1:19) Bronsted acid 170℃,30 min 78.5 0.502 [24] [BMIM]Cl AlCl3 160℃,1.5 min 82.2 0.526 [25] 水/GVL(1:4) SAPO-18 (B/L=0.11) 205℃,40 min 95.1 0.609 [26] MIBK,pH=1.25 H+ 170℃,100 min 65.0 0.416 [27] 四氢呋喃(THF) HCl 160℃,60 min 87.0 0.557 [28] 水/甲苯(1:2) 硅铝磷酸盐(SAPO-44) 170℃,8 h 93.0 0.595 [29] 水/THF=(1:2) 0.48 mol/L HCl 164℃ 90.0 0.576 [30] 水/DCM(水/二氯甲烷)(1:2) 固体酸催化剂SCC 170℃,60 min 81.4 0.521 [31] 表 5 纤维素水解为葡萄糖的质量产率
Table 5. Yield of cellulose hydrolyzed to glucose
表 6 葡萄糖转化5-HMF的产率
Table 6. Yield of glucose converted to 5-HMF
液态环境 催化剂 反应条件 产率/% 质量产率/(g·g-1) 参考文献 水-diglyme(1:3) ZrP 180℃,2 h 61.0 0.427 [34] 水-diglyme(1:3) ZrP 180℃,3 h 63.0 0.441 [34] Lewis+Bronsted m-ZrP 155℃,6 h 46.1 0.323 [35] [EMIM]Cl CrCl3 100℃,3 h 68.0 0.476 [36] [EMIM]Br Al2O3-b-0.05 140℃,3 h 49.7 0.348 [37] DMSO SnPCP@MnO2-PDA 150℃,5 h 55.8 0.391 [38] 表 7 葡萄糖转化LA的产率
Table 7. Yield of glucose converted to levulinic acid
液态环境 催化剂 反应条件 产率/% 质量产率/(g·g-1) 参考文献 水/GVL(1:4) 0.3 mol/L H2SO4 160℃,90 min 64.1 0.413 [39] 40 g/L葡萄糖 磷钨酸银盐0.7 g/25 mL 200℃,2 h 81.6 0.526 [40] 水/GVL(1:4) SAPO-18 (B/L=0.17) 180℃,80 min 70.2 0.452 [26] 秸秆/水(1:15) S2O82-/ZrO2-SiO2-Sm2O3: 13.3% 200℃,10 min 70.0 0.451 [41] 玉米芯浓度25 g/L SnCl4 80 mmol/L 193℃,10 min 78.1 0.503 [42] 表 8 阿拉伯糖转化糠醛的产率
Table 8. Yield of arabinose converted to furfural
表 9 蒸汽提取法热量消耗
Table 9. Heat consumption by steam extraction method
单元名称 单元说明 使用模块 热量消耗/(kJ·kg-1) STEAM-1 蒸汽生产单元1 FURNACE 16 796.20 FF-PRO 糠醛生产单元 RStoic -1 009.50 CON1 糠醛浓缩单元 Sep 15 490.80 STEAM-2 蒸汽生产单元2 FURNACE 19 314.20 LA-PRO LA生产单元 RStoic -1 237.87 NEU CaO中和单元 Sep 984.00 CON2 LA浓缩单元 Sep 17 331.40 注:热量消耗为在此工艺条件下每千克玉米秸秆所消耗的热量。 表 10 预处理-催化转化法热量消耗
Table 10. Heat consumption by pretreatment-catalytic conversion method
单元名称 单元说明 使用模块 热量消耗/(kJ·kg-1) HCL-PRE 稀盐酸预处理 RStoic 1 473.57 MEMB 木糖浓缩单元 Sep 91.89 FF-PRO 糠醛生成单元 RStoic 695.69 DIST1 糠醛浓缩单元 Sep 701.72 GLU-PRO&LA-PRO LA生产单元 RStoic 1 463.75 DIST2 LA浓缩单元 Sep 4 402.86 注:热量消耗为在此工艺条件下每千克玉米秸秆所消耗的热量。 表 11 两级预处理转化方法热量消耗
Table 11. Heat consumption by two-stage pretreatment conversion method
单元名称 单元说明 使用模块 热量消耗/(kJ·kg-1) LOW-T&FF-PRO 低温反应单元 RStoic 639.13 HIGH-T&LA-PRO 高温反应单元 RStoic 688.32 CO2 超临界CO2萃取 Sep 0 SEP2 蒸馏提取单元 Sep 1 255.86 注:热量消耗为在此工艺条件下每千克玉米秸秆所消耗的热量。 表 12 预处理后制备糠醛和5-HMF的热消耗
Table 12. Heat consumption for production of furfural and 5-HMF after pretreatment
单元名称 单元说明 使用模块 热量消耗/(kJ·kg-1) HCl-PRE 稀盐酸预处理 RStoic 1 463.66 MEMB1 木糖浓缩单元 Sep 91.88 FF-PRO 糠醛生成单元 RStoic 695.69 DIST1 糠醛浓缩单元 Sep 701.72 GLU-PRO 石灰处理单元 RStoic 178.74 MEMB2 葡萄糖浓缩单元 Sep 105.26 HMF-PRO 5-HMF生成单元 RStoic 1 075.67 DIST2 5-HMF浓缩单元 Sep 900.25 注:热量消耗为在此工艺条件下每千克玉米秸秆所消耗的热量。 -
[1] LI C Z, ZHAO X C, WANG A Q, et al. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chemical Reviews, 2015, 115(21): 11559-11624. doi: 10.1021/acs.chemrev.5b00155 [2] CHHEDA J N, DUMESIC J A. An overview of dehydration, aldol-condensation and hydrogenation processes for the production of liquid alkanes from biomass-derived carbohydrates[J]. ChemInform, 2007, 38(39): 59-70. [3] 赵蒙蒙, 姜曼, 周祚万. 几种农作物秸秆的成分分析[J]. 材料导报, 2011, 25(16): 122-125. https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201116034.htmZHAO M M, JIANG M, ZHOU Z W. The components analysis of several kinds of agricultural residues[J]. Materials Review, 2011, 25(16): 122-125(in Chinese). https://www.cnki.com.cn/Article/CJFDTOTAL-CLDB201116034.htm [4] SHI N, LIU Q Y, ZHANG Q, et al. High yield production of 5-hydroxymethylfurfural from cellulose by high concentration of sulfates in biphasic system[J]. Green Chemistry, 2013, 15(7): 1967. doi: 10.1039/c3gc40667a [5] 李丹, 文飚, 曹春昱. 植物原料中半纤维素预水解反应动力学及其机理研究进展[J]. 中国造纸, 2019, 38(2): 61-66. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZZ201902016.htmLI D, WEN B, CAO C Y. Research progress on hemicellulose pre-hydrolysis kinetics and mechanism of plant materials[J]. China Pulp & Paper, 2019, 38(2): 61-66(in Chinese). https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZZ201902016.htm [6] 龙金星, 徐莹, 王铁军, 等. 木质素催化解聚与氢解[J]. 新能源进展, 2014, 2(2): 83-88. doi: 10.3969/j.issn.2095-560X.2014.02.001LONG J X, XU Y, WANG T J, et al. Catalytic depolymerization and hydrogenolysis of lignin[J]. Advances in New and Renewable Energy, 2014, 2(2): 83-88(in Chinese). doi: 10.3969/j.issn.2095-560X.2014.02.001 [7] HEITNER C, DIMMEL D, SCHMIDT J. Lignin and lignans: Advances in chemistry[M]. Boca Raton: CRC Press, 2010. [8] VASSILEV S V, BAXTER D, ANDERSEN L K, et al. An overview of the organic and inorganic phase composition of biomass[J]. Fuel, 2012, 94: 1-33. [9] ENOMOTO K, HOSOYA T, MIYAFUJI H. High-yield production of 5-hydroxymethylfurfural from d-fructose, d-glucose, and cellulose by its in situ removal from the reaction system[J]. Cellulose, 2018, 25(4): 2249-2257. doi: 10.1007/s10570-018-1717-3 [10] 张琦, 李宇萍, 陈伦刚, 等. 百吨/年规模生物质水相合成航油类烃过程的物质与能量转化[J]. 天津大学学报(自然科学与工程技术版), 2017, 50(1): 13-18. https://www.cnki.com.cn/Article/CJFDTOTAL-TJDX201701003.htmZHANG Q, LI Y P, CHEN L G, et al. Material and energy conversion of integrated 100 t/a-scale bio-jet fuel-range hydrocarbon production system via aqueous conversion of biomass[J]. Journal of Tianjin University (Science and Technology), 2017, 50(1): 13-18(in Chinese). https://www.cnki.com.cn/Article/CJFDTOTAL-TJDX201701003.htm [11] 许文娟. 木质纤维素类生物质水解制取糠醛及乙酰丙酸的研究[D]. 上海: 华东理工大学, 2016.XU W J. Study on the production of furfural and levulinic acid from lignocellulosic biomass[D]. Shanghai: East China University of Science and Technology, 2016(in Chinese). [12] PENG P, PENG F, BIAN J, et al. Isolation and structural characterization of hemicelluloses from the bamboo species Phyllostachys incarnata Wen[J]. Carbohydrate Polymers, 2011, 86(2): 883-890. doi: 10.1016/j.carbpol.2011.05.038 [13] XU F, LIU C F, GENG Z C, et al. Characterisation of degraded organosolv hemicelluloses from wheat straw[J]. Polymer Degradation and Stability, 2006, 91(8): 1880-1886. doi: 10.1016/j.polymdegradstab.2005.11.002 [14] 袁梅婷, 翟华敏, 冯年捷, 等. 麦草自水解过程中半纤维素和木质素的变化特性[J]. 纤维素科学与技术, 2015, 23(2): 55-61. doi: 10.3969/j.issn.1004-8405.2015.02.009YUAN M T, ZHAI H M, FENG N J, et al. Characterization of hemicelluloses and lignin change of wheat straw in autohydrolysis process[J]. Journal of Cellulose Science and Technology, 2015, 23(2): 55-61(in Chinese). doi: 10.3969/j.issn.1004-8405.2015.02.009 [15] SHUAI L, QUESTELL-SANTIAGO Y M, LUTERBACHER J S. A mild biomass pretreatment using γ-valerolactone for concentrated sugar production[J]. Green Chemistry, 2016, 18(4): 937-943. doi: 10.1039/C5GC02489G [16] 赵绘婷, 刘振, 任秋鹤, 等. 玉米秸秆纤维素高效分离工艺研究[J]. 河南科学, 2019, 37(8): 1328-1333. doi: 10.3969/j.issn.1004-3918.2019.08.021ZHAO H T, LIU Z, REN Q H, et al. Efficient separation technique of cellulose from corn straw[J]. Henan Science, 2019, 37(8): 1328-1333(in Chinese). doi: 10.3969/j.issn.1004-3918.2019.08.021 [17] 张循海, 宋贺明, 贾宏葛, 等. 漆酶系统提取玉米秸秆中的纤维素[J]. 齐齐哈尔大学学报(自然科学版), 2020, 36(5): 43-44. doi: 10.3969/j.issn.1007-984X.2020.05.010ZHANG X H, SONG H M, JIA H G, et al. The laccase extracting cellulose systems from corn straw[J]. Journal of Qiqihar University (Natural Science Edition), 2020, 36(5): 43-44(in Chinese). doi: 10.3969/j.issn.1007-984X.2020.05.010 [18] 杨文玲, 王妨茶. 玉米秸秆纤维素提取工艺优化[J]. 安徽农业科学, 2019, 47(1): 198-201. doi: 10.3969/j.issn.0517-6611.2019.01.058YANG W L, WANG F C. Optimization of cellulose extraction process of corn straw[J]. Journal of Anhui Agricultural Sciences, 2019, 47(1): 198-201(in Chinese). doi: 10.3969/j.issn.0517-6611.2019.01.058 [19] ZU S, LI W Z, ZHANG M J, et al. Pretreatment of corn stover for sugar production using dilute hydrochloric acid followed by lime[J]. Bioresource Technology, 2014, 152: 364-370. doi: 10.1016/j.biortech.2013.11.034 [20] 张扬, 王运红, 邓立红, 等. 稻秸半纤维素水解条件和水解液脱毒的研究[J]. 纤维素科学与技术, 2005, 13(2): 38-44. doi: 10.3969/j.issn.1004-8405.2005.02.007ZHANG Y, WANG Y H, DENG L H, et al. Study of hemicellulose hydrolysis of the rice straw and hydrolysate detoxification[J]. Journal of Cellulose Science and Technology, 2005, 13(2): 38-44(in Chinese). doi: 10.3969/j.issn.1004-8405.2005.02.007 [21] LIU L, SUN J S, LI M, et al. Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3pretreatment[J]. Bioresource Technology, 2009, 100(23): 5853-5858. doi: 10.1016/j.biortech.2009.06.040 [22] 高文中, 张佳, 付存亭, 等. 玉米秸秆的水热酸处理工艺技术研究[J]. 化工设计通讯, 2019, 45(8): 80-81. doi: 10.3969/j.issn.1003-6490.2019.08.054GAO W Z, ZHANG J, FU C T, et al. Study on hydrothermal acid treatment of corn stover[J]. Chemical Engineering Design Communications, 2019, 45(8): 80-81(in Chinese). doi: 10.3969/j.issn.1003-6490.2019.08.054 [23] XU Z P, LI W Z, DU Z J, et al. Conversion of corn stalk into furfural using a novel heterogeneous strong acid catalyst in γ-valerolactone[J]. Bioresource Technology, 2015, 198: 764-771. doi: 10.1016/j.biortech.2015.09.104 [24] ZHANG T W, LI W Z, XU Z P, et al. Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone[J]. Bioresource Technology, 2016, 209: 108-114. doi: 10.1016/j.biortech.2016.02.108 [25] ZHANG L X, YU H B, WANG P, et al. Conversion of xylan, D-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid[J]. Bioresource Technology, 2013, 130: 110-116. doi: 10.1016/j.biortech.2012.12.018 [26] LI X Y, XU R, LIU Q L, et al. Valorization of corn stover into furfural and levulinic acid over SAPO-18 zeolites: Effect of Brønsted to Lewis acid sites ratios[J]. Industrial Crops and Products, 2019, 141: 111759. [27] KAUR I, NI Y H. A process to produce furfural and acetic acid from pre-hydrolysis liquor of kraft based dissolving pulp process[J]. Separation and Purification Technology, 2015, 146: 121-126. [28] XING R, SUBRAHMANYAM A V, OLCAY H, et al. Production of jet and diesel fuel range alkanes from waste hemicellulose-derived aqueous solutions[J]. Green Chemistry, 2010, 12(11): 1933. doi: 10.1039/c0gc00263a [29] BHAUMIK P, DHEPE P L. Exceptionally high yields of furfural from assorted raw biomass over solid acids[J]. RSC Advances, 2014, 4(50): 26215. [30] XING R, QI W, HUBER G W. Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries[J]. Energy & Environmental Science, 2011, 4(6): 2193. [31] 邓奥杰. 玉米芯两步法高效制备糠醛的研究[D]. 广州: 华南理工大学, 2016.DENG A J. Study on high-efficiency preparation of furfural from corncob by two-step method[D]. Guangzhou: South China University of Technology, 2016(in Chinese). [32] HOU X D, FENG G J, YE M, et al. Significantly enhanced enzymatic hydrolysis of rice straw via a high-performance two-stage deep eutectic solvents synergistic pretreatment[J]. Bioresource Technology, 2017, 238: 139-146. [33] CHEN L H, CHEN R, FU S Y. FeCl3 pretreatment of three lignocellulosic biomass for ethanol production[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(8): 1794-1800. [34] JAIN A, SHORE A M, JONNALAGADDA S C, et al. Conversion of fructose, glucose and sucrose to 5-hydroxymethyl-2-furfural over mesoporous zirconium phosphate catalyst[J]. Applied Catalysis A: General, 2015, 489: 72-76. [35] SARAVANAN K, PARK K S, JEON S, et al. Aqueous phase synthesis of 5-hydroxymethylfurfural from glucose over large pore mesoporous zirconium phosphates: Effect of calcination temperature[J]. ACS Omega, 2018, 3(1): 808-820. [36] ZHAO H B, HOLLADAY J E, BROWN H, et al. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural[J]. Science, 2007, 316(5831): 1597-1600. [37] HOU Q D, ZHEN M N, LI W Z, et al. Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid[J]. Applied Catalysis B: Environmental, 2019, 253: 1-10. [38] LI K, DU M M, JI P J. Multifunctional tin-based heterogeneous catalyst for catalytic conversion of glucose to 5-hydroxymethylfurfural[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(4): 5636-5644. [39] LI M H, LI W Z, LIU Q Y, et al. A two-step conversion of corn stover into furfural and levulinic acid in a water/gamma-valerolactone system[J]. BioResources, 2016, 11(4): 8239-8256. [40] 曾珊珊, 林鹿, 刘娣, 等. 磷钨酸盐催化转化葡萄糖合成乙酰丙酸[J]. 化工学报, 2012, 63(12): 3875-3881. https://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201212025.htmZENG S S, LIN L, LIU D, et al. Catalytic conversion of glucose to levulinic acid by solid heteropolyacid salts[J]. CIESC Journal, 2012, 63(12): 3875-3881(in Chinese). https://www.cnki.com.cn/Article/CJFDTOTAL-HGSZ201212025.htm [41] CHEN H, YU B, JIN S. Production of levulinic acid from steam exploded rice straw via solid superacid, S2O82-/ZrO2-SiO2-Sm2O3[J]. Bioresource Technology, 2011, 102(3): 3568-3570. [42] 卿青, 郭琪, 周琳琳, 等. SnCl4催化玉米芯高效制备乙酰丙酸的工艺研究[J]. 常州大学学报(自然科学版), 2018, 30(2): 14-22. https://www.cnki.com.cn/Article/CJFDTOTAL-JSSY201802003.htmQING Q, GUO Q, ZHOU L L, et al. Study on high efficiency catalytical preparation of levulinic acid from corncob by SnCl4[J]. Journal of Changzhou University(Natural Science Edition), 2018, 30(2): 14-22(in Chinese). https://www.cnki.com.cn/Article/CJFDTOTAL-JSSY201802003.htm [43] ZHAO Y, XU H, LU K F, et al. Experimental and kinetic study of arabinose conversion to furfural in renewable butanone-water solvent mixture catalyzed by lewis acidic ionic liquid catalyst[J]. Industrial & Engineering Chemistry Research, 2019, 58(36): 17088-17097. [44] DUSSAN K, GIRISUTA B, LOPES M, et al. Conversion of hemicellulose sugars catalyzed by formic acid: Kinetics of the dehydration of D-xylose, l-arabinose, and D-glucose[J]. ChemSusChem, 2015, 8(8): 1411-1428. [45] HONGSIRI W, DANON B, DE JONG W. The effects of combined catalysis of oxalic acid and seawater on the kinetics of xylose and arabinose dehydration to furfural[J]. International Journal of Energy and Environmental Engineering, 2015, 6(1): 21-30. [46] LE GUENIC S, GERGELA D, CEBALLOS C, et al. Furfural production from d-xylose and xylan by using stable nafion NR50 and NaCl in a microwave-assisted biphasic reaction[J]. Molecules, 2016, 21(8): 1102. [47] LI Y P, CHEN L G, ZHANG X H, et al. Process and techno-economic analysis of bio-jet fuel-range hydrocarbon production from lignocellulosic biomass via aqueous phase deconstruction and catalytic conversion[J]. Energy Procedia, 2017, 105: 675-680. [48] 章茹, 刘辉, 冯斐. 低聚木糖的膜分离浓缩工艺研究[J]. 食品工业, 2012, 33(9): 26-29. https://www.cnki.com.cn/Article/CJFDTOTAL-SPGY201209010.htmZHANG R, LIU H, FENG F. Study on the concentration of xylooligosaccharides by membrane separation technology[J]. The Food Industry, 2012, 33(9): 26-29(in Chinese). https://www.cnki.com.cn/Article/CJFDTOTAL-SPGY201209010.htm -







 下载:
下载: