Effect of nitrate on exfoliation corrosion of 2A12-T4 aluminum alloy under full-immersion corrosion condition
-
摘要:
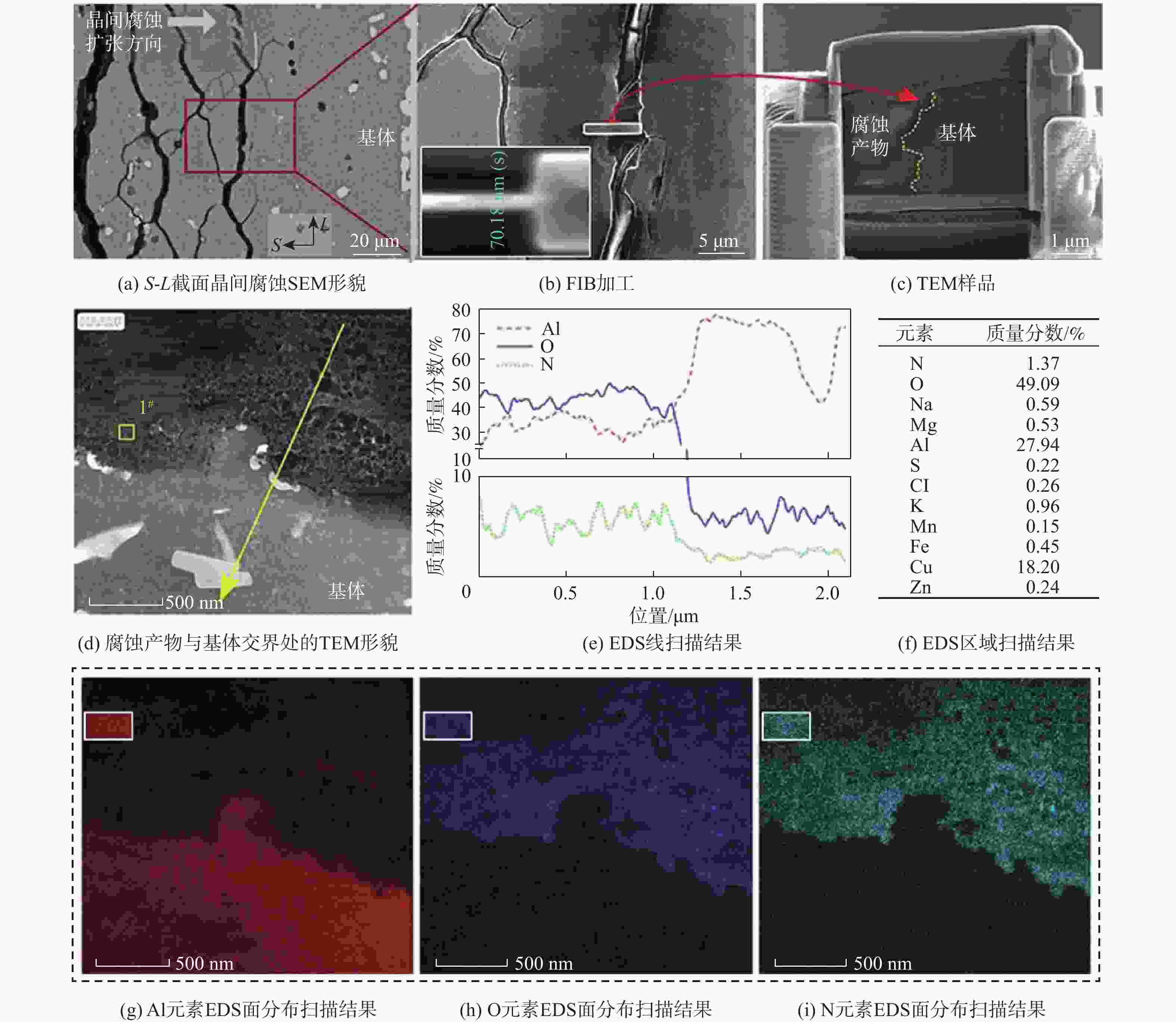
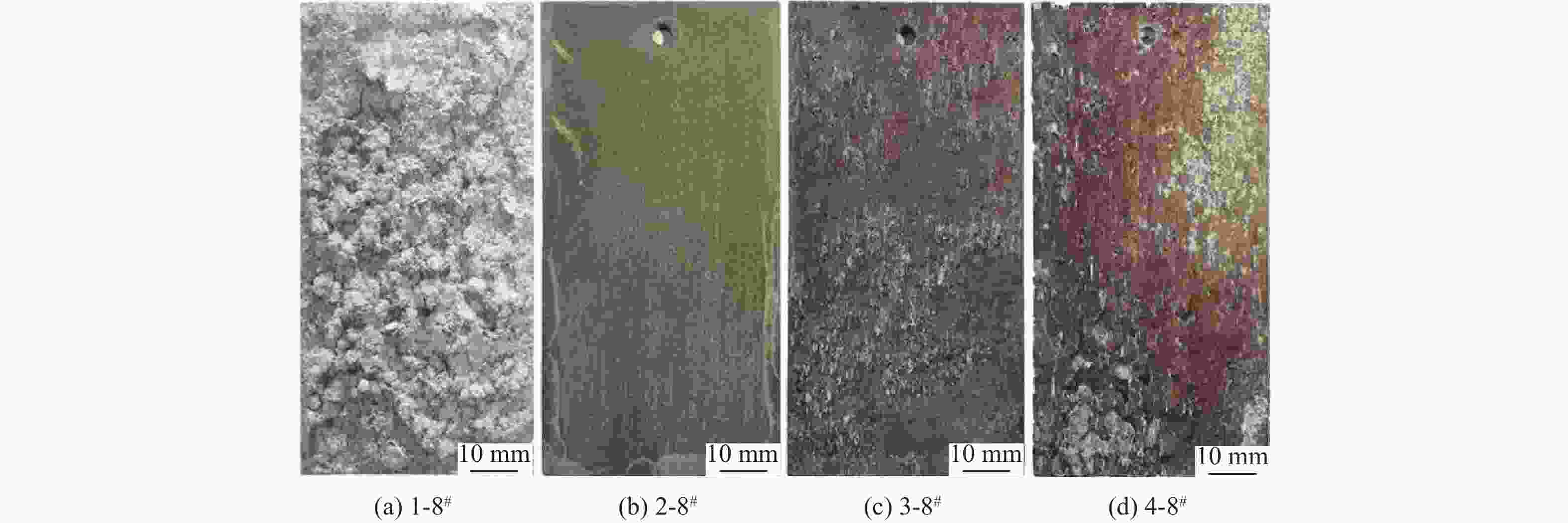
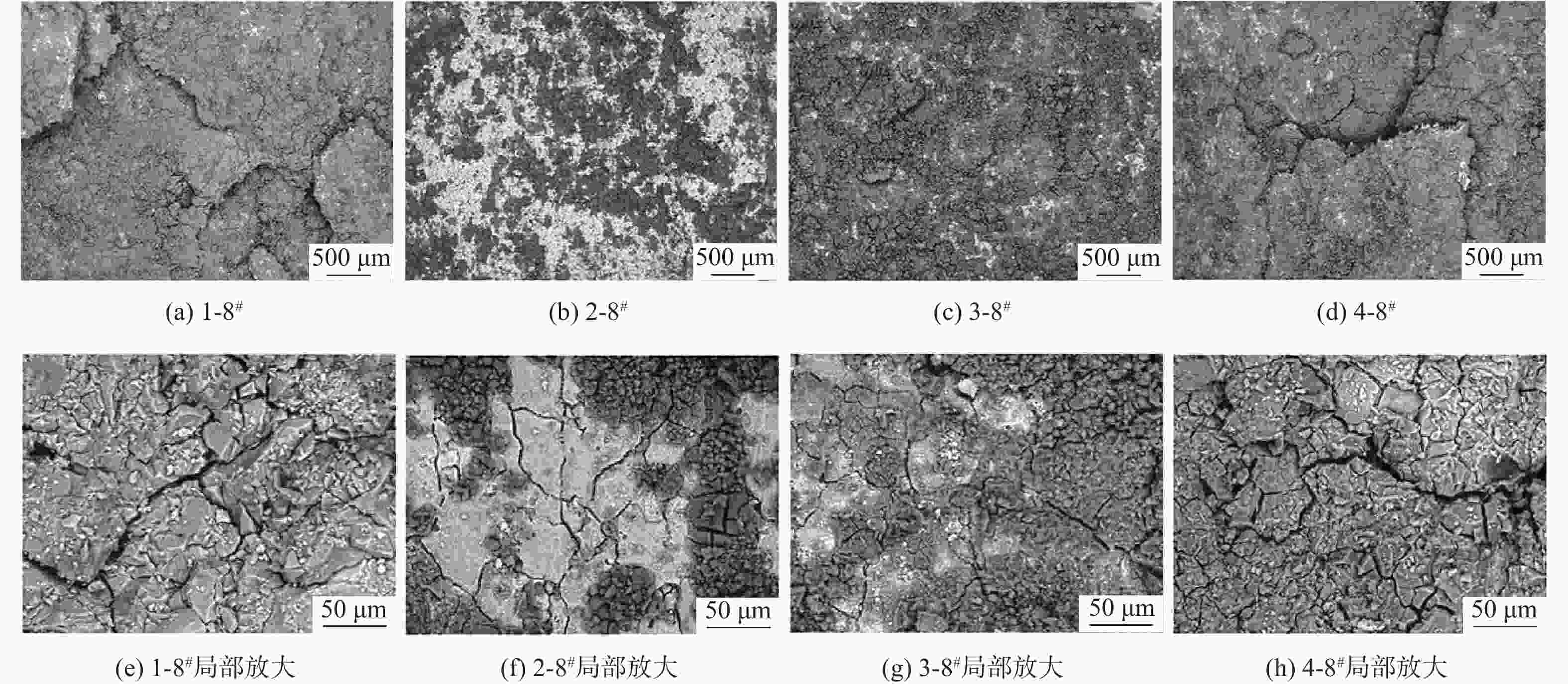
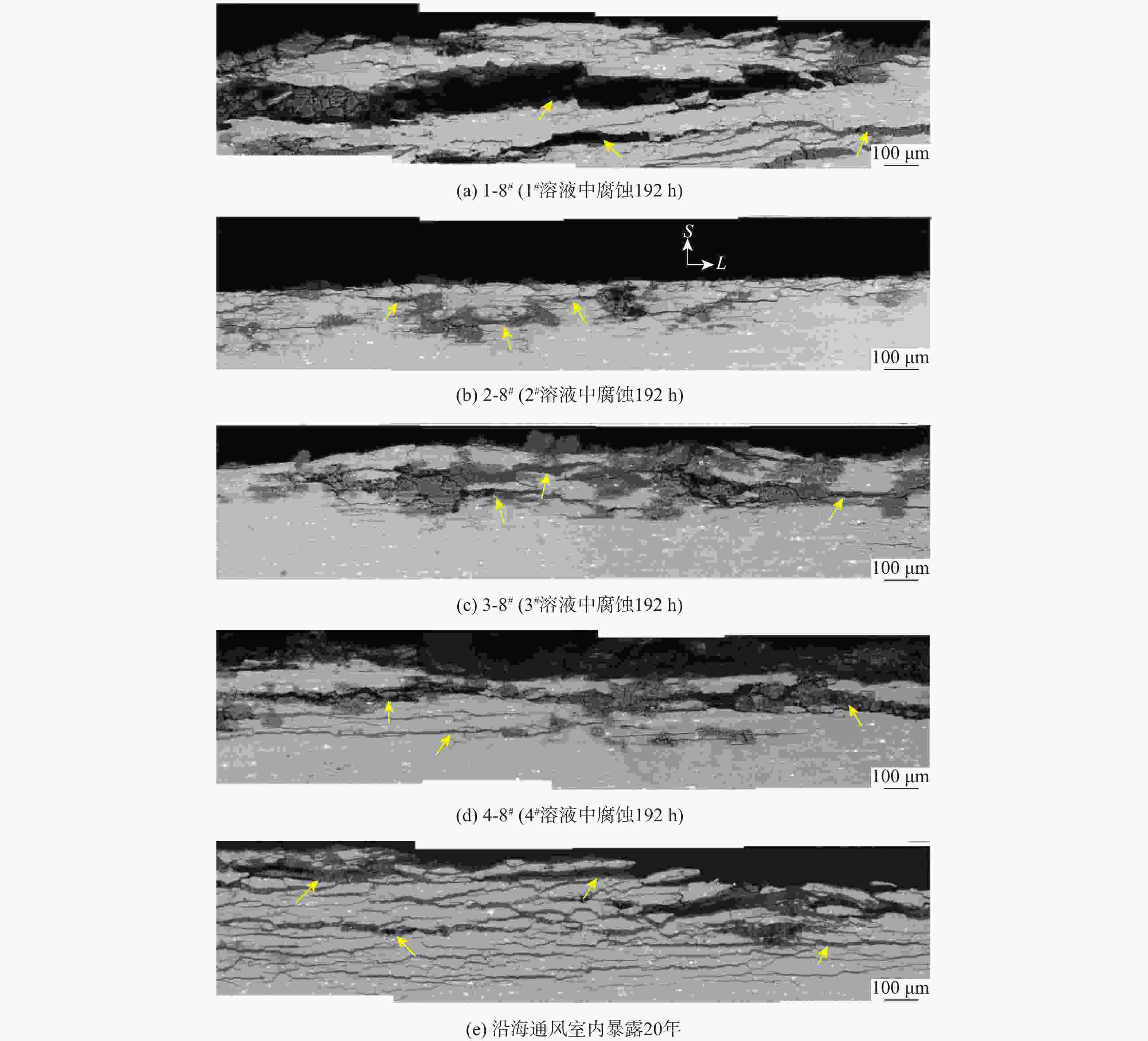
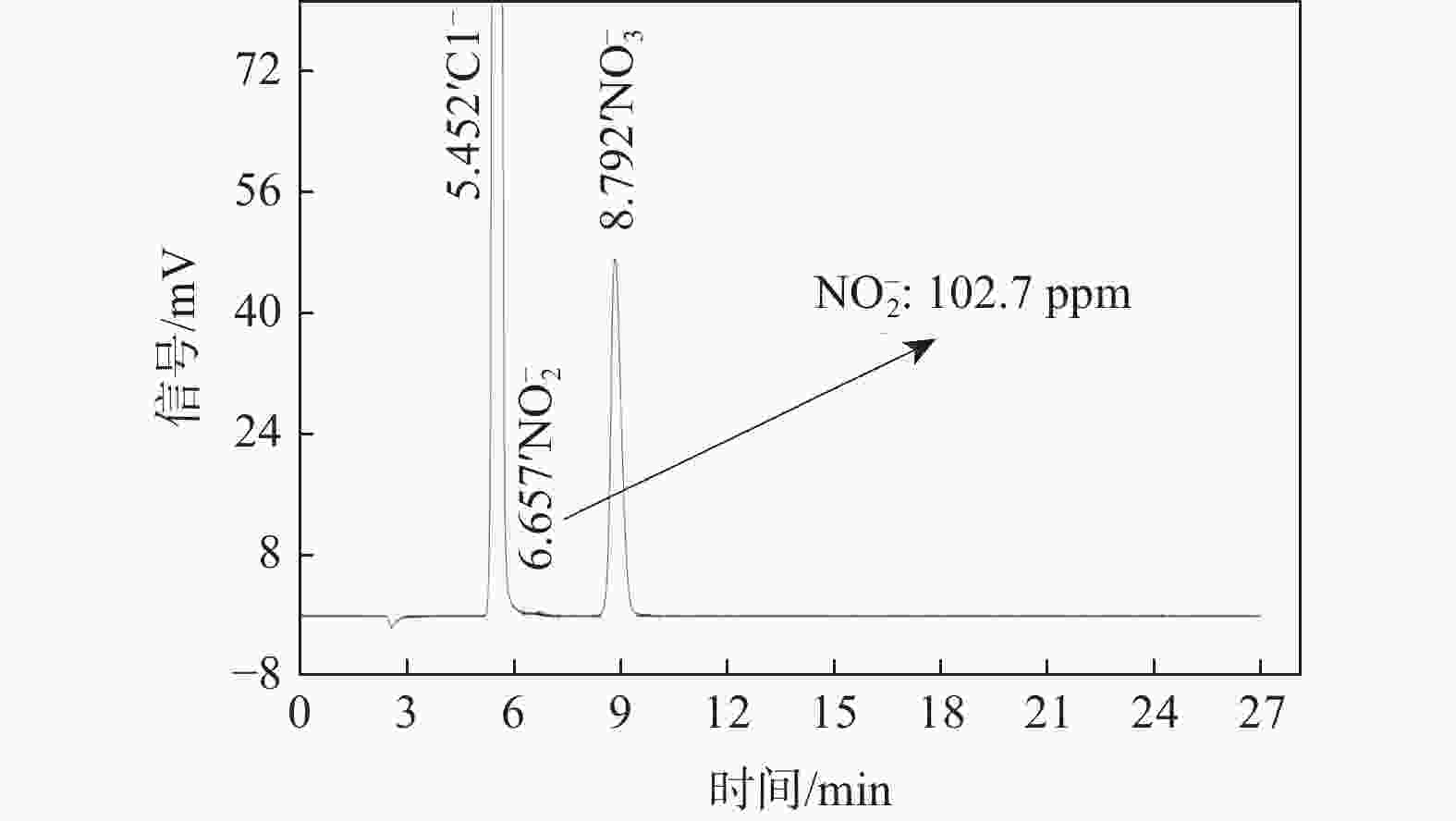
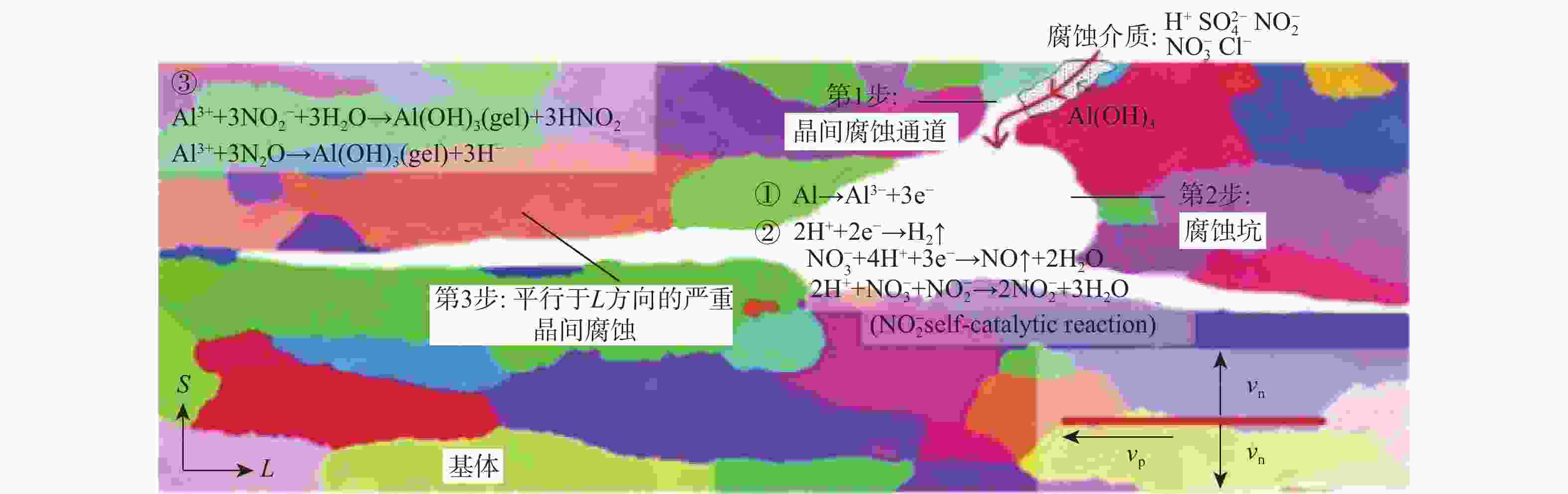
针对沿海暴露20年的2A12-T4铝合金试验件,采用球差校正透射电镜,在剥蚀区域纵截面腐蚀最深处的腐蚀产物中,首次测到了N元素的存在,说明铝合金沿海大气剥蚀过程中存在${\text{NO}}_x^{{ - }}$。开展2A12-T4铝合金在4种不同${\text{NO}}_3^{{ - }}$浓度腐蚀溶液中的全浸腐蚀试验,发现随着溶液中${\text{NO}}_3^{{ - }}$浓度降低,试验件的剥蚀严重程度明显减弱,且在含有${\text{Cl}}_{}^{{ - }}$和${\text{SO}}_4^{{{2 - }}}$,而不含${\text{NO}}_3^{{ - }}$的溶液中,试验件未能发生剥蚀。结合对产生气体的测试和离子色谱仪分析,表面和截面腐蚀行为扫描电子显微镜(SEM)、能谱仪(EDS)分析等,讨论${\text{NO}}_3^{{ - }}$对铝合金剥蚀的影响机理。建议在开展飞机铝合金结构沿海大气剥蚀模拟试验时,不能忽视腐蚀环境中${\text{NO}}_3^{{ - }}$的作用。
Abstract:The 2A12-T4 aluminum alloy specimen exposed to the coastal atmosphere for 20 years was analyzed with spherical aberration corrected transmission electron microscope. The presence of nitrogen was first determined in the corrosion products at the deepest corrosion part of the longitudinal section of the exfoliation corrosion area. This indicated the presence of ${\text{NO}}_x^{{ - }}$ in the coastal atmospheric exfoliation corrosion process of aluminum alloy. Then the full immersion corrosion tests of 2A12-T4 aluminum alloy in four different nitrate concentration corrosion solutions were carried out. It was found that the exfoliation corrosion severity of the specimens decreased significantly with the decrease of nitrate concentration in the solution; while in the solution containing ${\text{Cl}}_{}^{{ - }}$ and ${\text{SO}}_4^{{{2 - }}}$ rather than ${\text{NO}}_3^{{ - }}$, the exfoliation corrosion of the specimens did not occur. At the same time, combined with the test of the generated gas, ion chromatograph analysis, surface and sectional corrosion behavior SEM and EDS analysis, etc., the influence mechanism of nitrate on the exfoliation corrosion of aluminum alloy was analyzed and discussed. It is suggested that the role of nitrate in the corrosive environment cannot be ignored when carrying out the coastal atmospheric exfoliation corrosion simulation tests of aircraft aluminum alloy structures.
-
Key words:
- nitrogen element /
- exfoliation corrosion /
- aluminum alloy /
- nitrate /
- atmospheric exfoliation corrosion
-
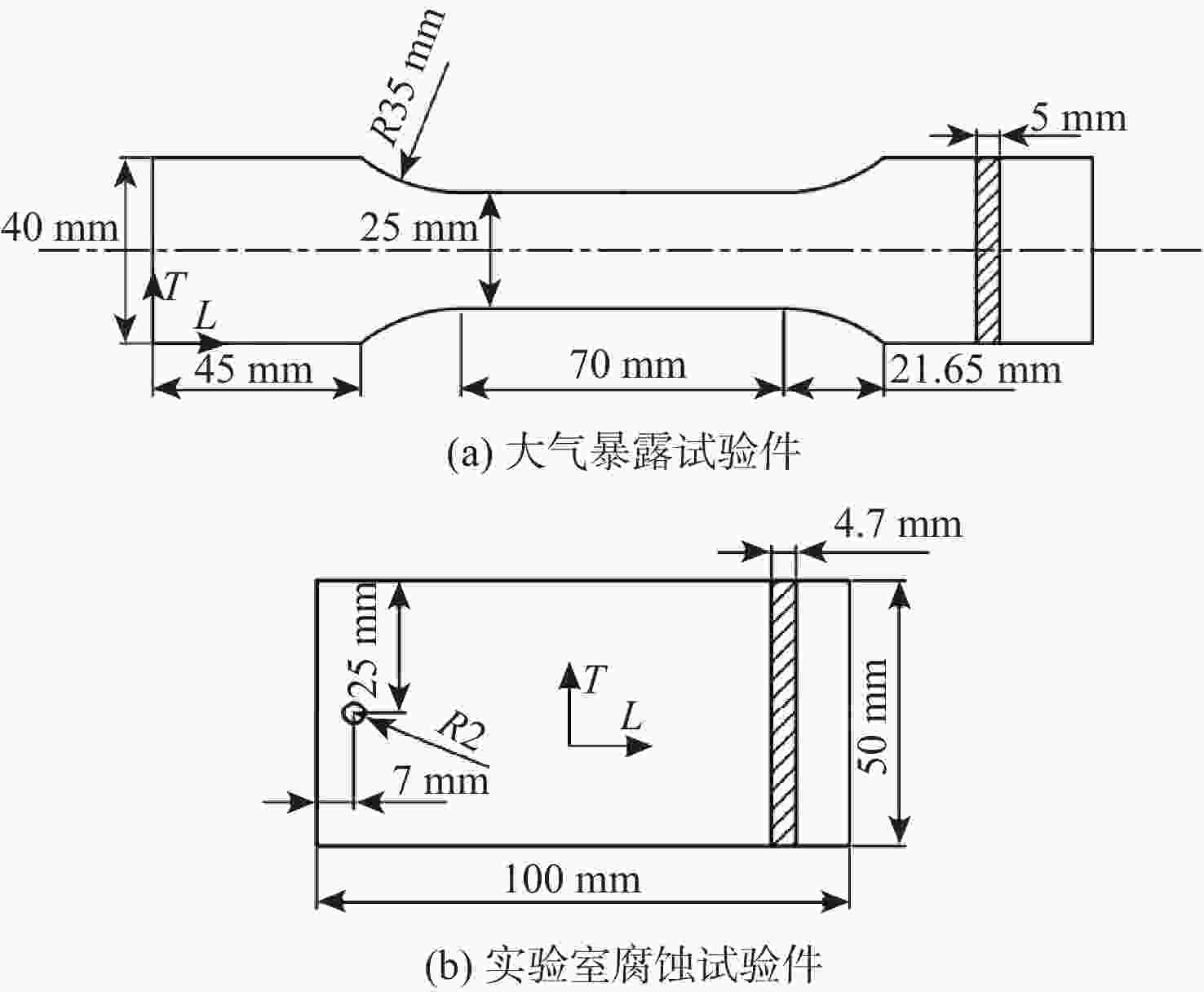
表 1 2类试验件2A12-T4铝合金材料的化学成分
Table 1. Chemical composition of two types of specimens (2A12-T4 aluminum alloy)
% 类型 化学成分的质量分数 Si Fe Cu Mn Mg Ni Zn Ti Al 大气
暴露0.10 0.22 4.62 0.54 1.60 0.99 0.22 0.13 余量 实验室腐蚀 0.40 0.35 4.30 0.70 1.40 0.08 0.20 0.13 余量 表 2 万宁市自然环境试验站大气环境数据
Table 2. Environmental data of Wanning test site
环境参数 年平均值 温度/°C 23.9 相对湿度/% 87.6 降水量/mm 198.156 风速/(m·s−1) 2.431 SO2/(mg·m−3) 0.045275 NO2/(mg·m−3) 0.002025 Cl− 沉积速率/(mg·(dm2·d)−1) 14.5875 降雨pH 5.125 表 3 不同腐蚀溶液腐蚀介质成分及浓度
Table 3. Components and concentrations of corrosion media in different corrosion solutions
腐蚀溶液编号 腐蚀介质成分 浓度/ (mol·L−1) PH 1#(EXCO溶液) NaCl 4.00 0.4 KNO3 0.50 HNO3 0.10 2# NaCl 4.00 0.4 K2SO4 0.25 H2SO4 0.05 3# NaCl 4.00 0.4 K2SO4 0.25 HNO3 0.10 4# NaCl 4.00 0.4 KNO3 0.50 H2SO4 0.05 表 4 试验件编号示例
Table 4. Example of specimen number
腐蚀
时间/h试验件编号 1#
(EXCO溶液)2#
(EXCO溶液)3#
(EXCO溶液)4#
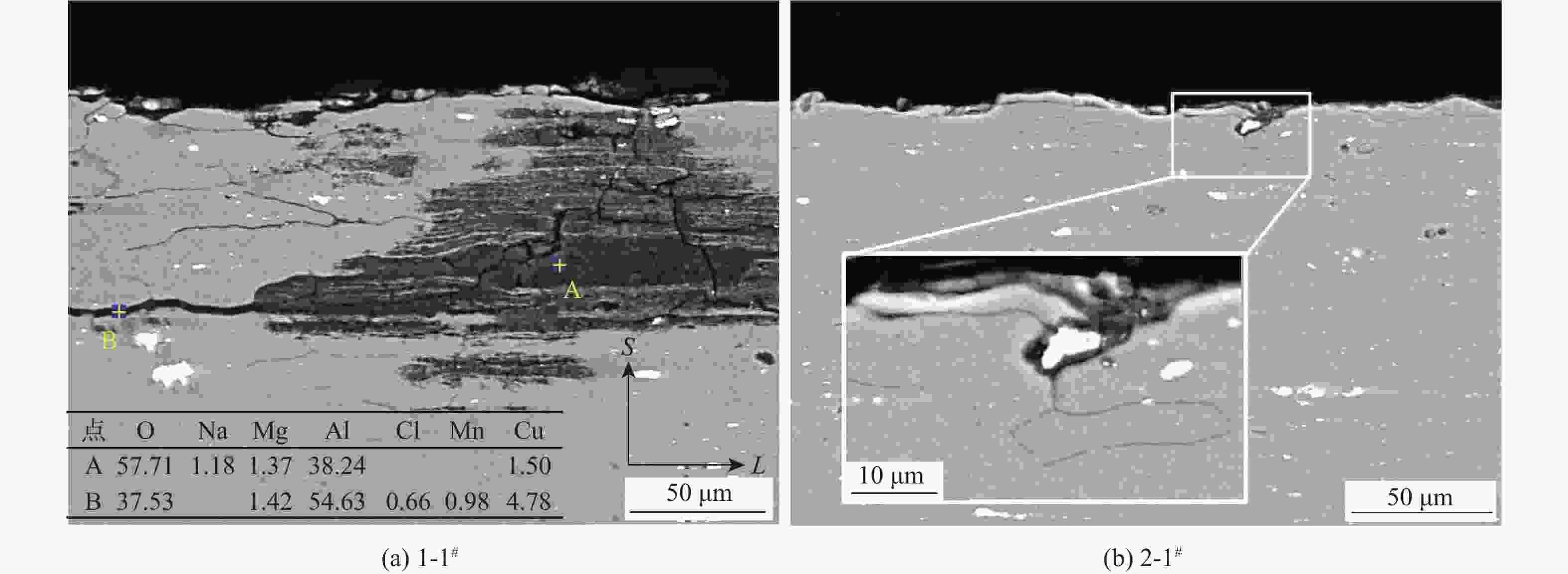
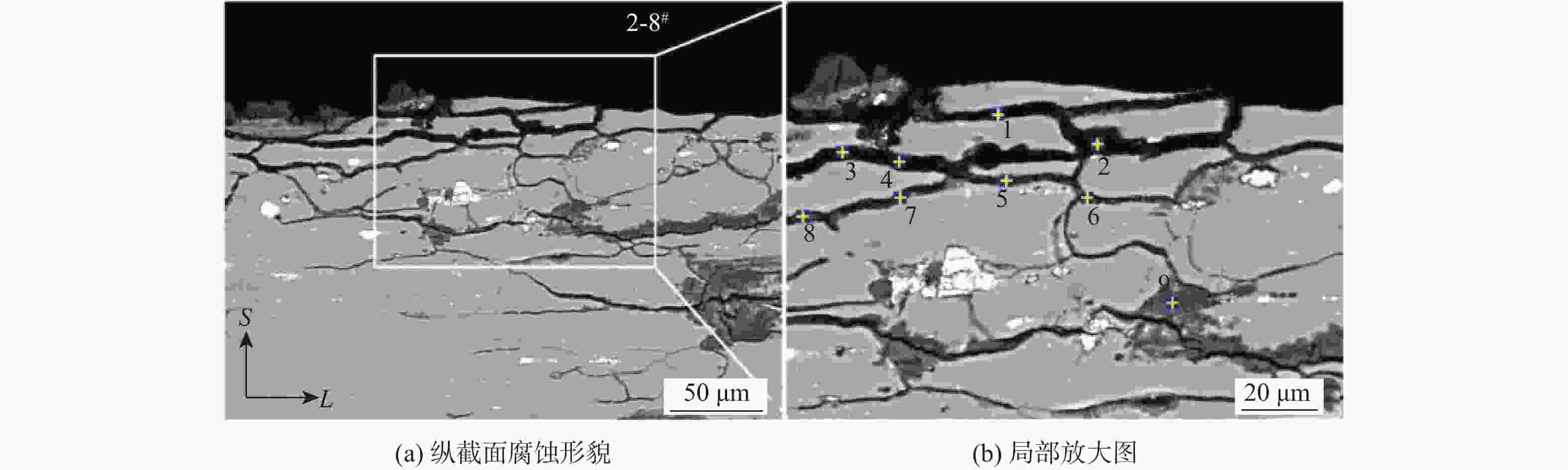
(EXCO溶液)5 1-1# 2-1# 3-1# 4-1# 24 1-2# 2-2# 3-2# 4-2# 48 1-3# 2-3# 3-3# 4-3# 96 1-4# 2-4# 3-4# 4-4# 120 1-5# 2-5# 3-5# 4-5# 144 1-6# 2-6# 3-6# 4-6# 168 1-7# 2-7# 3-7# 4-7# 192 1-8# 2-8# 3-8# 4-8# 表 5 2-8#试验件的纵截面(S-L)EDS数据
Table 5. EDS results of longitudinal S-L section of 2-8# specimen
% 点 化学成分质量分数 O Na Mg Al S Cl K Cu 1 20.34 1.08 68.55 10.03 2 24.45 1.82 64.44 9.30 3 17.29 1.49 61.86 19.36 4 29.67 1.21 59.70 9.42 5 33.84 0.95 0.92 58.57 1.82 0.37 3.53 6 47.75 1.03 45.36 2.45 3.41 7 40.88 1.07 0.62 51.66 1.60 0.61 0.41 3.14 8 45.10 0.87 47.94 2.17 0.67 0.32 2.93 9 60.46 32.09 3.08 0.66 3.71 -
[1] 李荻, 郭宝兰, 牟雅楠. 高强铝合金剥蚀及晶间腐蚀敏感性研究[J]. 中国有色金属学报, 2002, 12: 204-207.LI D, GUO B L, MOU Y N. Sensitivities to exfoliation and intergranular corrosion of high-strength aluminum alloys[J]. The Chinese of Nonferrous Metals, 2002, 12: 204-207(in Chinese). [2] ZHANG S, ZHANG T, HE Y, et al. Long-term atmospheric corrosion of aluminum alloy 2024-T4 in coastal environment: Surface and sectional corrosion behavior[J]. Journal of Alloys and Compounds, 2019, 789: 460-471. doi: 10.1016/j.jallcom.2019.03.028 [3] LIAO M, RENAUD G, BELLINGER N C. Fatigue modeling for aircraft structures containing natural exfoliation corrosion[J]. International Journal of Fatigue, 2007, 29(4): 677-686. doi: 10.1016/j.ijfatigue.2006.07.003 [4] 安百刚, 张学元, 韩恩厚, 等. 铝和铝合金的大气腐蚀研究现状[J]. 中国有色金属学报, 2001, 11(S2): 11-15. doi: 10.3321/j.issn:1004-0609.2001.z2.003AN B G, ZHANG X Y, HAN E H, et al. Research situation of atmospheric corrosion of aluminum and aluminum alloys[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(S2): 11-15(in Chinese). doi: 10.3321/j.issn:1004-0609.2001.z2.003 [5] MENDOZA A R, CORVO F. Outdoor and indoor atmospheric corrosion of non-ferrous metals[J]. Corrosion Science, 2000, 42(7): 1123-1147. doi: 10.1016/S0010-938X(99)00135-3 [6] CASTAÑO J G, FUENTE D, MORCILLO M. A laboratory study of the effect of NO2 on the atmospheric corrosion of zinc[J]. Atmospheric Environment, 2007, 41(38): 8681-8696. doi: 10.1016/j.atmosenv.2007.07.022 [7] KOUDELKOVA M, AUGUSTYNSKI J. Some aspects of the anodic behavior of aluminum in nitrate-chloride solutions[J]. Journal of the Electrochemical Society, 1979, 126(10): 1659-1661. doi: 10.1149/1.2128771 [8] 李晓刚. 材料腐蚀与防护概论[M]. 北京: 机械工业出版社, 2017.LI X G. Introduction to material corrosion and protection[M]. Beijing: China Machine Press, 2017(in Chinese). [9] ADAMS A A, EAGLE K E, FOLEY R T. Synergistic effects of anions in the corrosion of aluminum alloys[J]. Journal of The Electrochemical Society, 1972, 119(12): 1692-1694. doi: 10.1149/1.2404072 [10] MCINTYRE J F, DOW T S. Intergranular corrosion behavior of aluminum alloys exposed to artificial seawater in the presence of nitrate anion[J]. Corrosion, 1992, 48(4): 309-319. doi: 10.5006/1.3315937 [11] KEDDAM M, KUNTZ C, TAKENOUTI H, et al. Exfoliation corrosion of aluminum alloys examined by electrode impedance[J]. Electrochimica Acta, 1997, 42(1): 87-97. doi: 10.1016/0013-4686(96)00170-3 [12] CAO F H, ZHANG Z, LI J F, et al. Exfoliation corrosion of aluminum alloy AA7075 examined by electrochemical impedance spectroscopy[J]. Materials and Corrosion, 2004, 55(1): 18-23. doi: 10.1002/maco.200303691 [13] XIAO Y P, PAN Q L, LI W B, et al. Exfoliation corrosion of Al-Zn-Mg-Cu-Zr alloy containing Sc examined by electrochemical impedance spectroscopy[J]. Materials and Corrosion, 2012, 63(2): 127-133. doi: 10.1002/maco.201005696 [14] PARKER M E, KELLY R G. Investigating the impact of accelerated testing variables on the exfoliation corrosion of AA2060[J]. Corrosion, 2016, 72(11): 1342-1350. doi: 10.5006/2103 [15] International Organization for Standardization. 2011 metals and alloys-atmospheric corrosion testing - general requirements: ISO 8565[S]. Geneva: International Organization for Standardization, 2011. [16] 戴树桂. 环境化学[M]. 北京: 高等教育出版社, 2006.DAI S G. Environmental chemistry[M]. Beijing: Higher Education Press, 2006(in Chinese). [17] ZHANG S, ZHANG T, HE Y, et al. Long-term atmospheric pre-corrosion fatigue properties of epoxy primer-coated 7075-T6 aluminum alloy structures[J]. International Journal of Fatigue, 2019, 129: 105225. doi: 10.1016/j.ijfatigue.2019.105225 [18] American Society of Testing Materials. Standard test method for exfoliation corrosion susceptibility in 2XXX and 7XXX series aluminum alloys (EXCO test): ASTM G34-01 [S]. West Conshohocken: ASTM International, 2013. [19] SPROWLS D O, WALSH J D, SHUMAKER M B. Simplified exfoliation testing of aluminum alloys[J]. American Society for Testing and Materials, 1972: 38-65. [20] ROBINSON M J. The role of wedging stresses in the exfoliation corrosion of high strength aluminum alloys[J]. Corrosion Science, 1983, 23(8): 887-899. doi: 10.1016/0010-938X(83)90016-1 [21] 王俊杰. 硝酸和金属反应产物与硝酸氧化性强弱关系分析[J]. 内蒙古电大学刊, 2000(5): 35.WANG J J. Analysis of the relationship between the reaction product of nitric acid and metal and the strength of oxidizing ability of nitric acid[J]. Journal of Inner Mongolia Radio & TV University, 2000(5): 35(in Chinese). [22] 张淑民. 基础无机化学(上册)[M]. 兰州: 兰州大学出版社, 2013.ZHANG S M. Basic inorganic chemistry (Volume I)[M]. Lanzhou: Lanzhou University Press, 2013(in Chinese). [23] 李毅, 廖霞, 肖仁贵, 等. 硝酸根离子对铝箔直流电蚀电极反应过程的影响[J]. 轻合金加工技术, 2016, 44(8): 66-71.LI Y, LIAO X, XIAO R G, et al. The influence of nitrate ion on electrode reaction process of aluminum foil DC etching[J]. Light Alloy Fabrication Technology, 2016, 44(8): 66-71(in Chinese). [24] 李芳芳. 高强铝合金剥落腐蚀的研究综述[J]. 湖南冶金职业技术学院学报, 2009, 9(2): 9-12.LI F F. Study on exfoliation corrosion of high strength aluminum alloys[J]. Journal of Hunan Metallurgical Professional Technology College, 2009, 9(2): 9-12(in Chinese). [25] 张胜. 沿海大气环境下铝合金板件长期腐蚀行为与疲劳特性研究[D]. 西安: 空军工程大学, 2020.ZHANG S. Research on long-term corrosion behavior and fatigue characteristics of aluminum alloy plates in coastal atmospheric environment[D]. Xi’an: Air Force Engineering University, 2020(in Chinese). [26] 李劲风, 张昭, 曹发和, 等. LC4铝合金剥蚀及其电化学阻抗行为[J]. 中国有色金属学报, 2002, 12(6): 1189-1193.LI J F, ZHANG Z, CAO F H, et al. Exfoliation corrosion and electrochemical impedance behavior of LC4 alloy[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(6): 1189-1193(in Chinese). [27] 于美, 刘建华, 李松梅. 航空铝合金腐蚀防护与检测方法[M]. 北京: 科学出版社, 2017.YU M, LIU J H, LI S M. Aviation aluminum alloy corrosion protection and detection methods[M]. Beijing: Science Press, 2017(in Chinese). [28] 王凤平, 康万利, 敬和民. 腐蚀电化学原理、方法及应用[M]. 北京: 化学工业出版社, 2008.WANG F P, KANG W L, JING H M. Principle, method and application of corrosion electrochemistry[M]. Beijing: Chemical Industry Press, 2008(in Chinese). [29] MARLAUD T, MALKI B, DESCHAMPS A, et al. Electrochemical aspects of exfoliation corrosion of aluminium alloys: The effects of heat treatment[J]. Corrosion Science, 2011, 53(4): 1394-1400. doi: 10.1016/j.corsci.2011.01.010 [30] 王彬, 苏艳. 铝合金大气腐蚀行为及其防腐措施研究进展[J]. 装备环境工程, 2012, 9(2): 64-68. doi: 10.3969/j.issn.1672-9242.2012.02.016WANG B, SU Y. Research progress in atmospheric corrosion behavior and anticorrosion measures of aluminum alloy[J]. Equipment Environmental Engineering, 2012, 9(2): 64-68(in Chinese). doi: 10.3969/j.issn.1672-9242.2012.02.016 -






 下载:
下载: