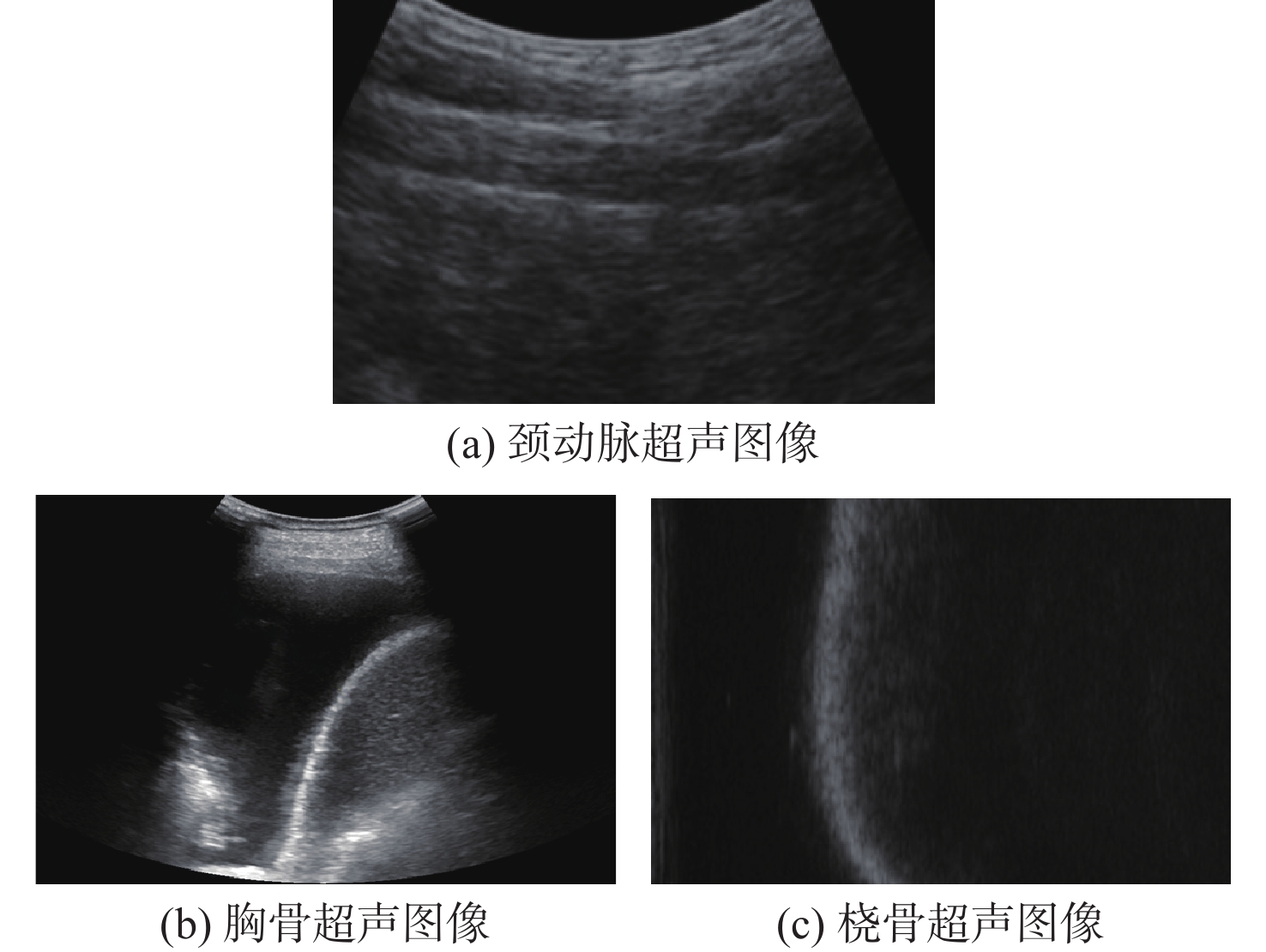
-
摘要:
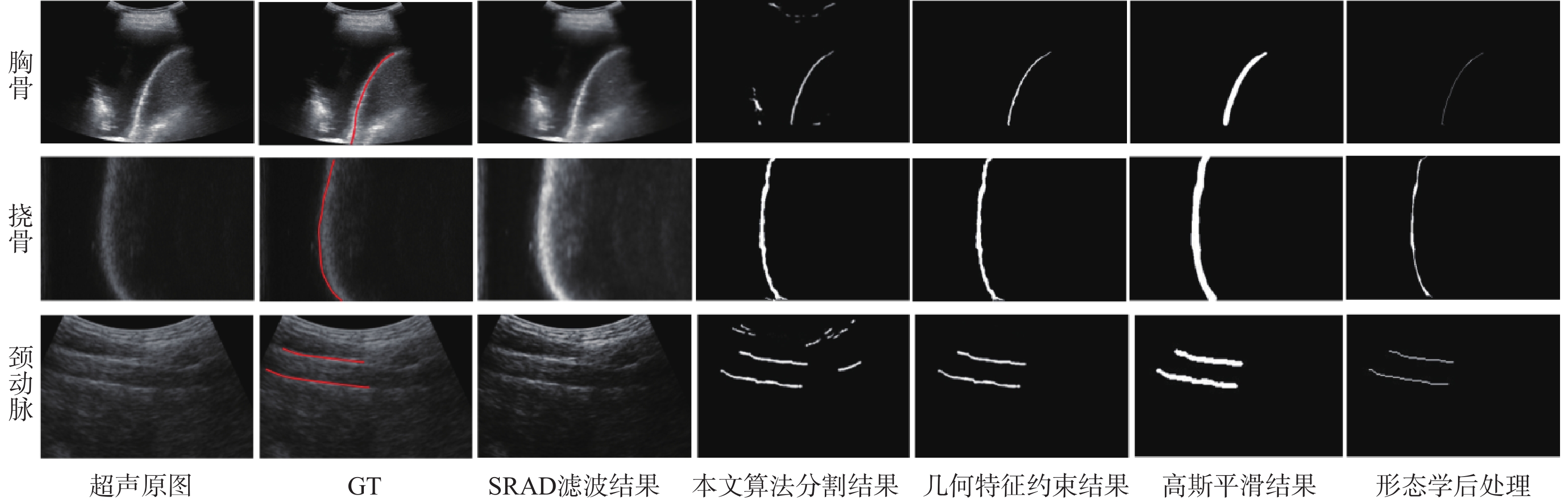
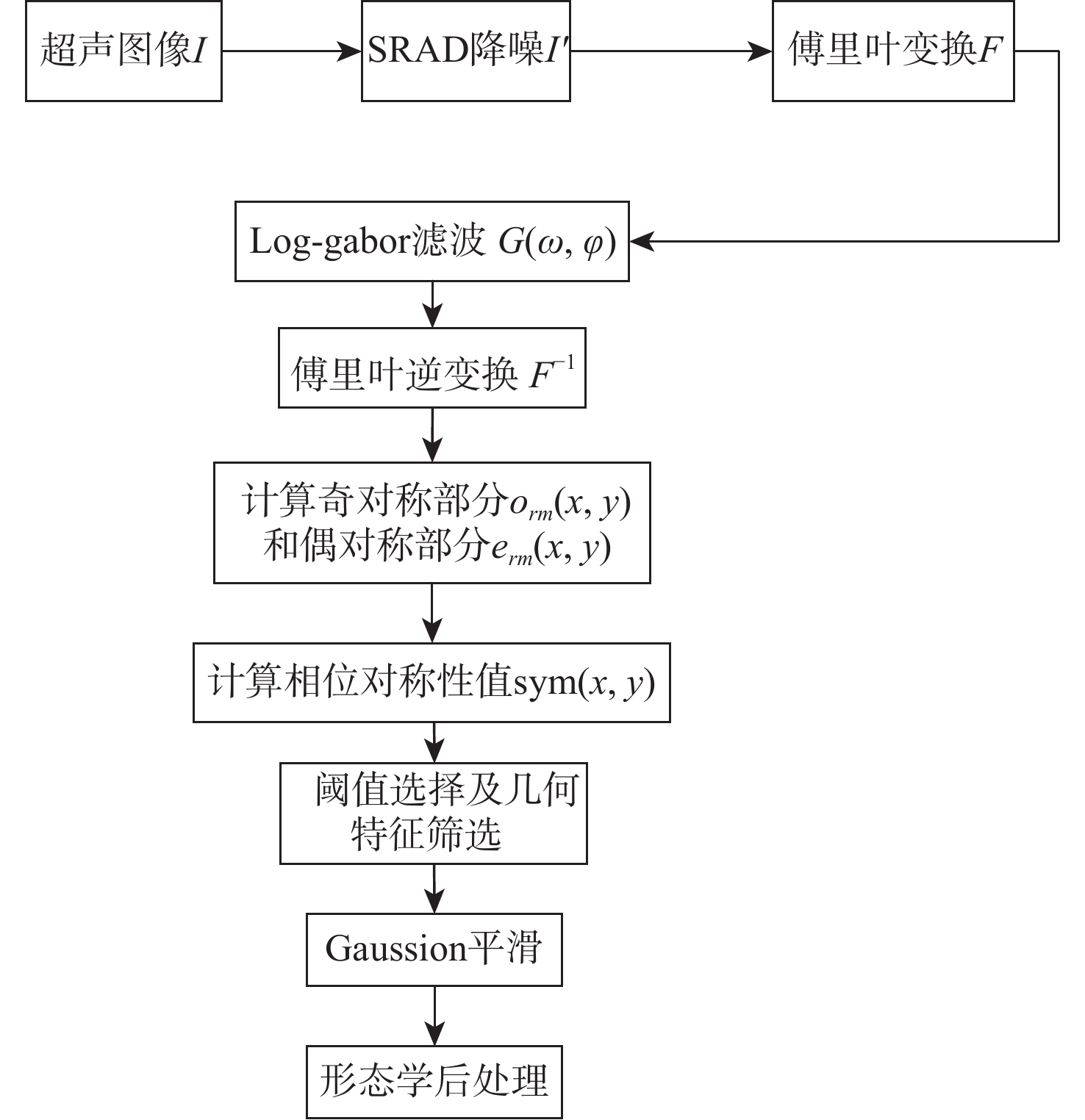
超声成像凭借其经济便携、无辐射、可实时成像等优点,已经成为临床诊断中常用的检查方法之一。血管超声成像不仅可以减少术中射线,也可以实现手术室外血管病变情况的初步判断,满足无法进行造影手术病人的检测需求。超声图像中目标组织边缘特征一般较为明显,综合考虑超声成像的特点及特征分布,选择基于相位对称性的分割算法对降噪滤波后超声图像中的目标组织进行分割,并根据目标组织边缘特性对分割结果进行形态学处理。通过与传统的活动轮廓型及深度学习算法对目标组织边缘的分割效果进行对比及量化分析,验证基于相位对称性的分割算法在目标组织分割及边缘提取中的优势。
Abstract:Ultrasound imaging has become one of the common examination methods in clinical diagnosis due to its advantages of economical, portable, radiation-free, and real-time imaging. Vascular ultrasound imaging can not only reduce intraoperative radiation but also realize the preliminary judgment of vascular lesions outside the operating room, meeting the detection needs of patients unable to undergo digital subtraction angiography. Edges of target tissues in the ultrasonic image are relatively obvious. Comprehensively considering ultrasonic image characteristics and feature distribution, this paper selects a phase symmetry segmentation algorithm to segment the target tissue edges of ultrasound images after noise filtering. Morphological processing is used to optimize the edges of segmentation results. The advantages of the phase symmetry algorithm proposed in this paper are verified by comparing the segmentation results with traditional active contour algorithm and a deep learning model.
-
Key words:
- ultrasonic imaging /
- image segmentation /
- noise reduction filtering /
- phase symmetry /
- deep learning
-
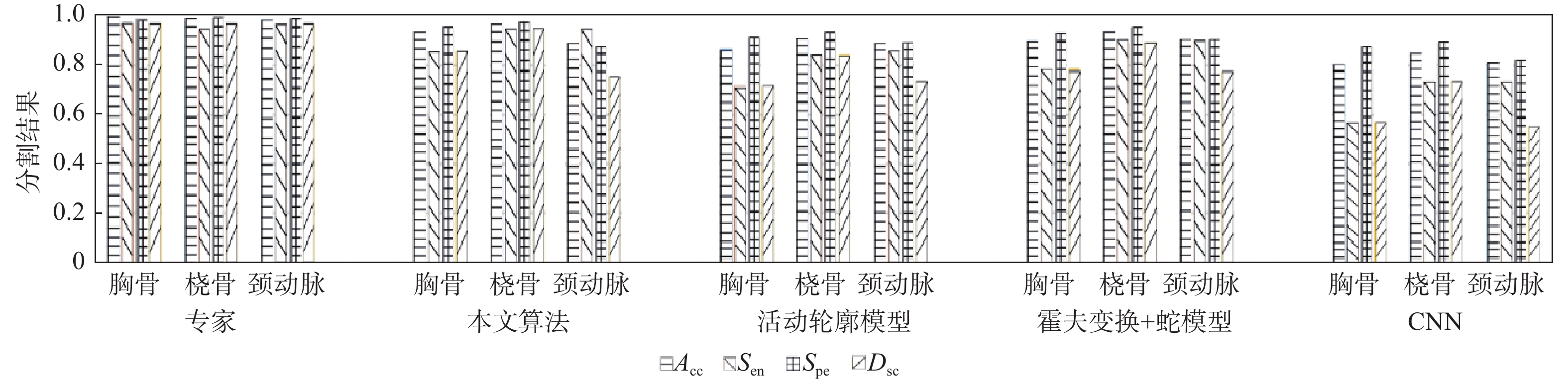
表 1 本文算法和专家分割结果对比
Table 1. Comparison of segmentation results between proposed algorithm and experts
算法 Acc Sen Spe Dsc 胸骨 桡骨 颈动脉 胸骨 桡骨 颈动脉 胸骨 桡骨 颈动脉 胸骨 桡骨 颈动脉 本文 0.93 0.97 0.89 0.86 0.95 0.95 0.95 0.98 0.88 0.86 0.95 0.75 专家 0.99 0.98 0.98 0.97 0.95 0.97 0.99 0.99 0.99 0.97 0.96 0.97 表 2 各算法平均运行时间
Table 2. Comparison of average running time of each algorithm
s 本文算法 活动轮廓模型 霍夫变换+蛇模型 CNN算法 2.57 12.63 15.57 0.50 -
[1] 傅向华. 2015年中国大陆冠心病介入数据发布[J]. 中国介入心脏病学杂志, 2016, 24(5): 276.FU X H. Data release of coronary heart disease intervention in Chinese mainland in 2015[J]. Chinese Journal of Interventional Cardiology, 2016, 24(5): 276(in Chinese). [2] 万明习. 生物医学超声学[M]. 北京: 科学出版社, 2010.WAN M X. Biomedical ultrasonics[M]. Beijing: Science Press, 2010 (in Chinese). [3] CHEN D, COHEN L D, MIREBEAU J M. Vessel extraction using anisotropic minimal paths and path score[C]//2014 IEEE International Conference on Image Processing. Piscataway: IEEE Press, 2015: 1570-1574. [4] AMIR-KHALILI A, HAMARNEH G, PEYRAT J M, et al. Automatic segmentation of occluded vasculature via pulsatile motion analysis in endoscopic robot-assisted partial nephrectomy video[J]. Medical Image Analysis, 2015, 25(1): 103-110. doi: 10.1016/j.media.2015.04.010 [5] SHIN S Y, LEE S, NOH K J, et al. Extraction of coronary vessels in fluoroscopic X-ray sequences using vessel correspondence optimization[C]//International Conference on Medical Image Computing and Computer-Assisted Intervention. Berlin: Springer, 2016: 308-316. [6] LEE B, YAN J Y, ZHUANG T G. A dynamic programming based algorithm for optimal edge detection in medical images[C]//Proceedings International Workshop on Medical Imaging and Augmented Reality. Piscataway: IEEE Press, 2002: 193-198. [7] 钱红莹. 基于改进Canny算子的医学图像边缘检测算法[J]. 软件导刊, 2019, 18(2): 45-48.QIAN H Y. A medical image edge detection algorithm based on improved canny operator[J]. Software Guide, 2019, 18(2): 45-48(in Chinese). [8] 侯格贤, 毕笃彦, 吴成柯. 图象分割质量评价方法研究[J]. 中国图象图形学报, 2000, 5(1): 39-43. doi: 10.3969/j.issn.1006-8961.2000.01.009HOU G X, BI D Y, WU C K. Researches on evaluation methods for image segmentation[J]. Journal of Image and Graphics, 2000, 5(1): 39-43(in Chinese). doi: 10.3969/j.issn.1006-8961.2000.01.009 [9] CREMERS D, ROUSSON M, DERICHE R. A review of statistical approaches to level set segmentation: integrating color, texture, motion and shape[J]. International Journal of Computer Vision, 2007, 72(2): 195-215. doi: 10.1007/s11263-006-8711-1 [10] LIU S F, WANG Y, YANG X, et al. Deep learning in medical ultrasound analysis: A review[J]. Engineering, 2019, 5(2): 261-275. doi: 10.1016/j.eng.2018.11.020 [11] AMIRI M, BROOKS R, RIVAZ H. Fine-tuning U-net for ultrasound image segmentation: Different layers, different outcomes[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2020, 67(12): 2510-2518. doi: 10.1109/TUFFC.2020.3015081 [12] CHEN C, QIN C, QIU H, et al. Deep learning for cardiac image segmentation: A review[J]. Frontiers in Cardiovascular Medicine, 2020, 7: 25. doi: 10.3389/fcvm.2020.00025 [13] CHEN Y R, LIU J, LUO X B, et al. ApodNet: Learning for high frame rate synthetic transmit aperture ultrasound imaging[J]. IEEE Transactions on Medical Imaging, 2021, 40(11): 3190-3204. doi: 10.1109/TMI.2021.3084821 [14] AL CHANTI D, DUQUE V G, CROUZIER M, et al. IFSS-net: Interactive few-shot Siamese network for faster muscle segmentation and propagation in volumetric ultrasound[J]. IEEE Transactions on Medical Imaging, 2021, 40(10): 2615-2628. doi: 10.1109/TMI.2021.3058303 [15] KOVESI P. Symmetry and asymmetry from local phase[C]//Tenth Australian Joint Conference on Artificial Intelligence, 1997: 190: 2-4. [16] HACIHALILOGLU I, ABUGHARBIEH R, HODGSON A J, et al. Automatic adaptive parameterization in local phase feature-based bone segmentation in ultrasound[J]. Ultrasound in Medicine & Biology, 2011, 37(10): 1689-1703. [17] YU Y J, ACTON S T. Speckle reducing anisotropic diffusion[J]. IEEE Transactions on Image Processing: A Publication of the IEEE Signal Processing Society, 2002, 11(11): 1260-1270. doi: 10.1109/TIP.2002.804276 [18] PETROUDI S, LOIZOU C, PANTZIARIS M, et al. Segmentation of the common carotid intima-media complex in ultrasound images using active contours[J]. IEEE Transactions on Biomedical Engineering, 2012, 59(11): 3060-3069. doi: 10.1109/TBME.2012.2214387 [19] XU X Y, ZHOU Y, CHENG X Y, et al. Ultrasound intima-media segmentation using Hough transform and dual snake model[J]. Computerized Medical Imaging and Graphics, 2012, 36(3): 248-258. doi: 10.1016/j.compmedimag.2011.06.007 [20] MENG C, SUN K, GUAN S Y, et al. Multiscale dense convolutional neural network for DSA cerebrovascular segmentation[J]. Neurocomputing, 2020, 373: 123-134. doi: 10.1016/j.neucom.2019.10.035 -







 下载:
下载: