-
摘要:
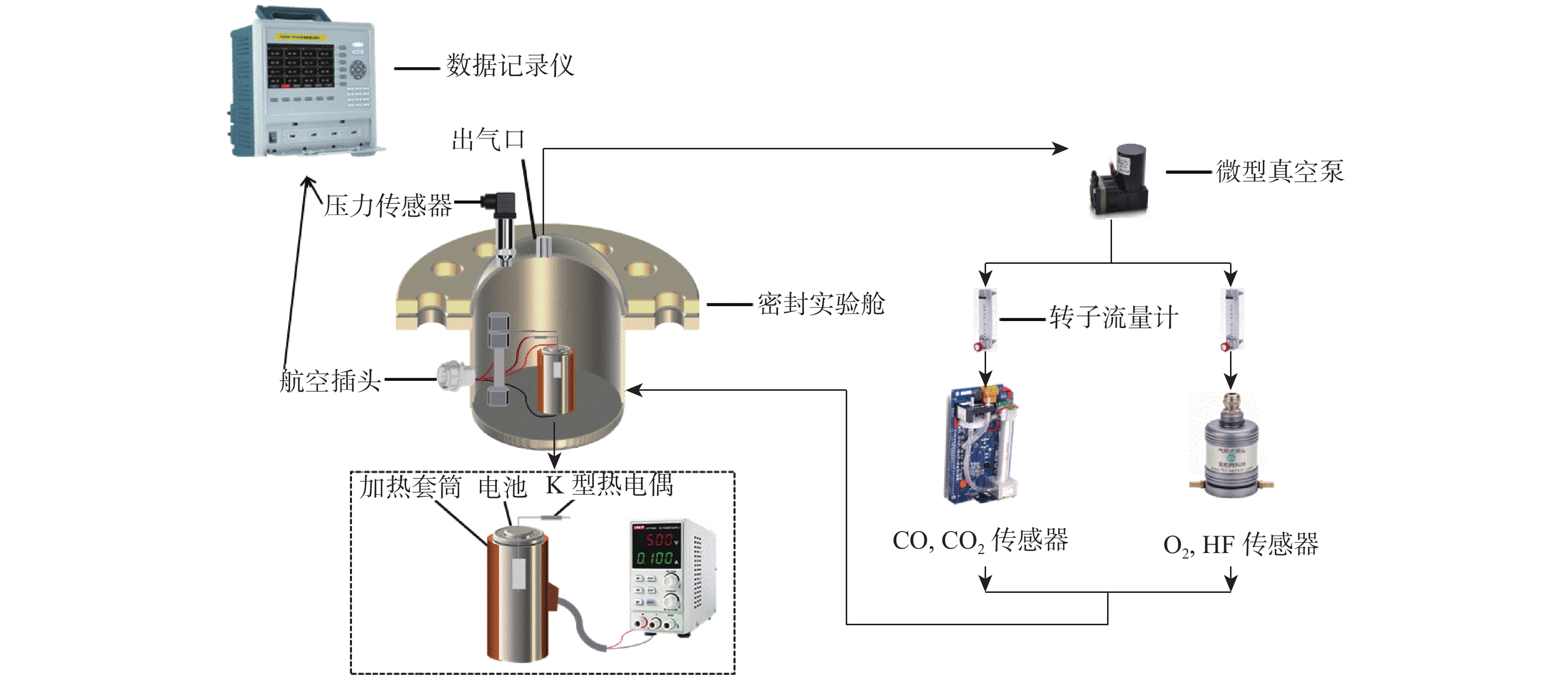
为研究锂离子电池热失控气体中主要有毒物质的危害程度,提出一种基于风险评估理论的锂离子电池热失控气体毒性风险分析方法。以点燃参数表征锂离子电池热失控发生概率,利用2种有效剂量分数(FED)方程与气体传感器阵列检测结果,建立锂离子电池热失控气体毒性动力学模型,进而表征气体毒性造成的后果,分析不同荷电状态(SOC)下三元锂离子电池热失控气体的毒性风险。结果表明:高SOC锂离子电池更易进入热失控状态,热失控释放的CO、HF及气体总量随SOC的增加而增加;锂离子电池SOC越高,热失控释放气体毒性风险越大,100% SOC锂离子电池毒性风险约为25% SOC锂离子电池的8倍,需要11倍的新鲜空气稀释才能达到安全浓度。研究结果可为锂离子电池热失控早期预警及气体毒性评价提供数据参考。
Abstract:A method to analyse the toxicity of thermal runaway gases of batteries, referring to the risk assessment theory was proposed. It aims to investigate the hazard ratings of the main harmful substances in the thermal runaway gas of lithium-ion batteries.By using the fractional effective dose (FED) equation and a gas sensor array, the results of the method used to characterize the likelihood of thermal runaway of lithium batteries occurred were discovered.The thermal runaway gas toxicity kinetic model of the battery was demonstrated in order to identify the consequences of gas toxicity, while the toxicity risk of thermal runaway gas in ternary lithium-ion batteries under different states of charge (SOC) was analyzed accordingly. The results retrieved show that high SOC batteries are more likely to enter the thermal runaway state, and the total amount of CO, HF and gas released by thermal runaway increases according to the SOC of batteries. As a result, the risk of thermal runaway increases with battery SOC.A fully (100%) charged battery has about 8 times the toxicity risk of a 25% charged battery and requires 11 times the fresh air dilution to reach a safe concentration.The results provided data reference for the early warning of lithium battery thermal runaway and the toxicity evaluation of pyrolysis gas.
-
Key words:
- ternary lithium-ion battery /
- thermal runaway /
- gas sensor array /
- risk assessment /
- toxicity analysis
-
表 1 实验电池参数
Table 1. Experimental battery parameters
参数 数值 高度/mm 65.1 直径/mm 18.5 额定容量/(mA·h) 3500 额定电压/V 3.635 两端电压/V 3.59±0.05(25% SOC) 3.72±0.05(50% SOC) 3.92±0.05(75% SOC) 4.16±0.05(100% SOC) 表 2 不同SOC电池关键参数
Table 2. Key parameters of batteries at different SOC
SOC Δt/s Tdrop/℃ Tvent/℃ TTR/℃ Tgmax/℃ Pmax/MPa P1/MPa dP/dtmax I 25% SOC 182±44 149.39±5.8 167.44±2.62 239.26±20.23 367.36±30.50 0.3158±0.127 0.1158±0.004 0.08248±0.062 2.86 50% SOC 140±10 144.47±2.88 162.29±3.23 224.26±6.25 396.86±23.46 0.2624±0.012 0.1267±0.003 0.08463±0.037 2.72 75% SOC 127±19 142.91±4.05 158.71±2.61 213.49±5.89 669.45±196.3 0.2653±0.059 0.1343±0.003 0.09264±0.033 2.66 100% SOC 105±19 133.78±1.30 148.14±3.50 193.86±4.33 868.36±334.4 0.3112±0.067 0.1378±0.003 0.10216±0.037 2.53 表 3 不同SOC释放气体量
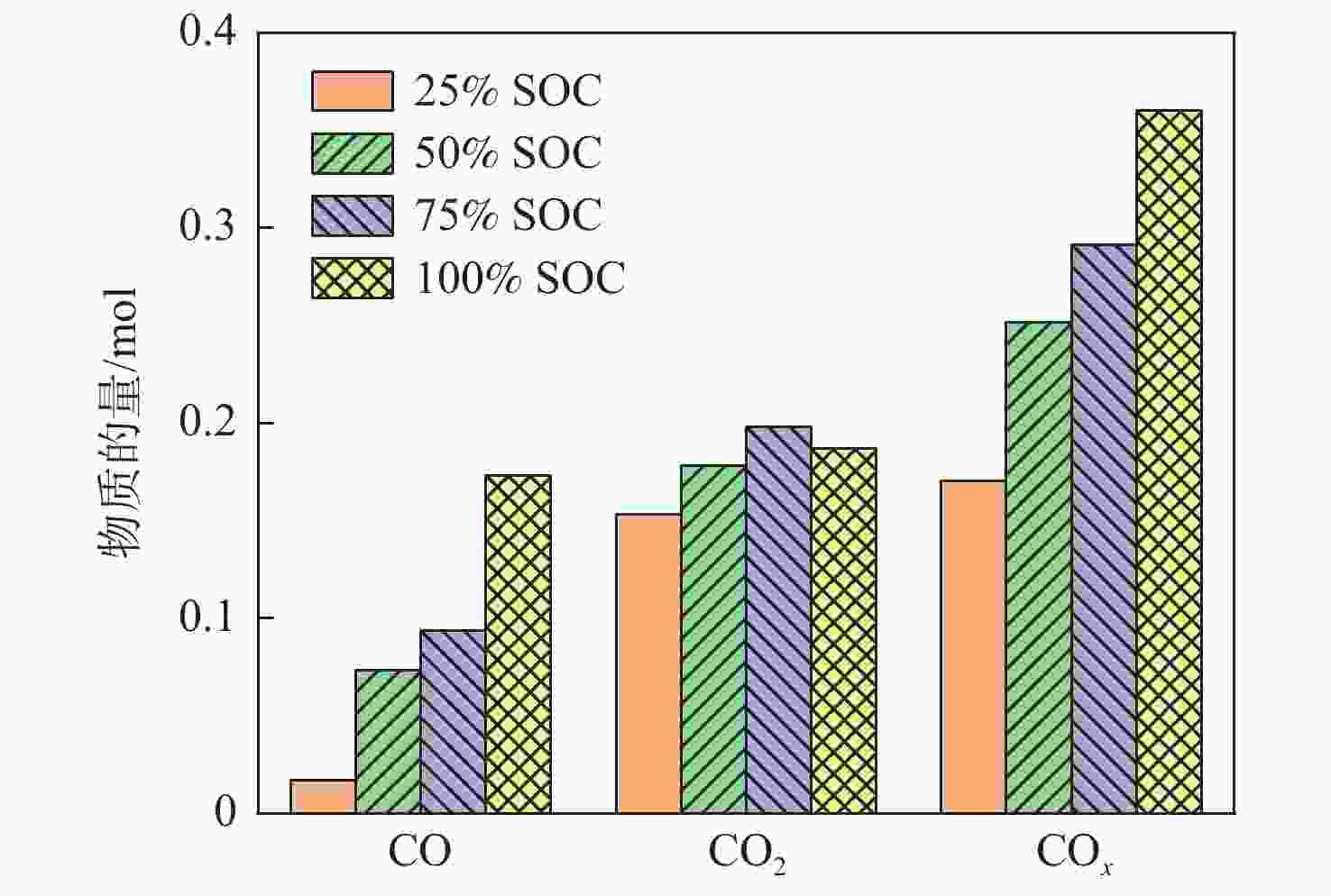
Table 3. Amountof vented gases at different SOC
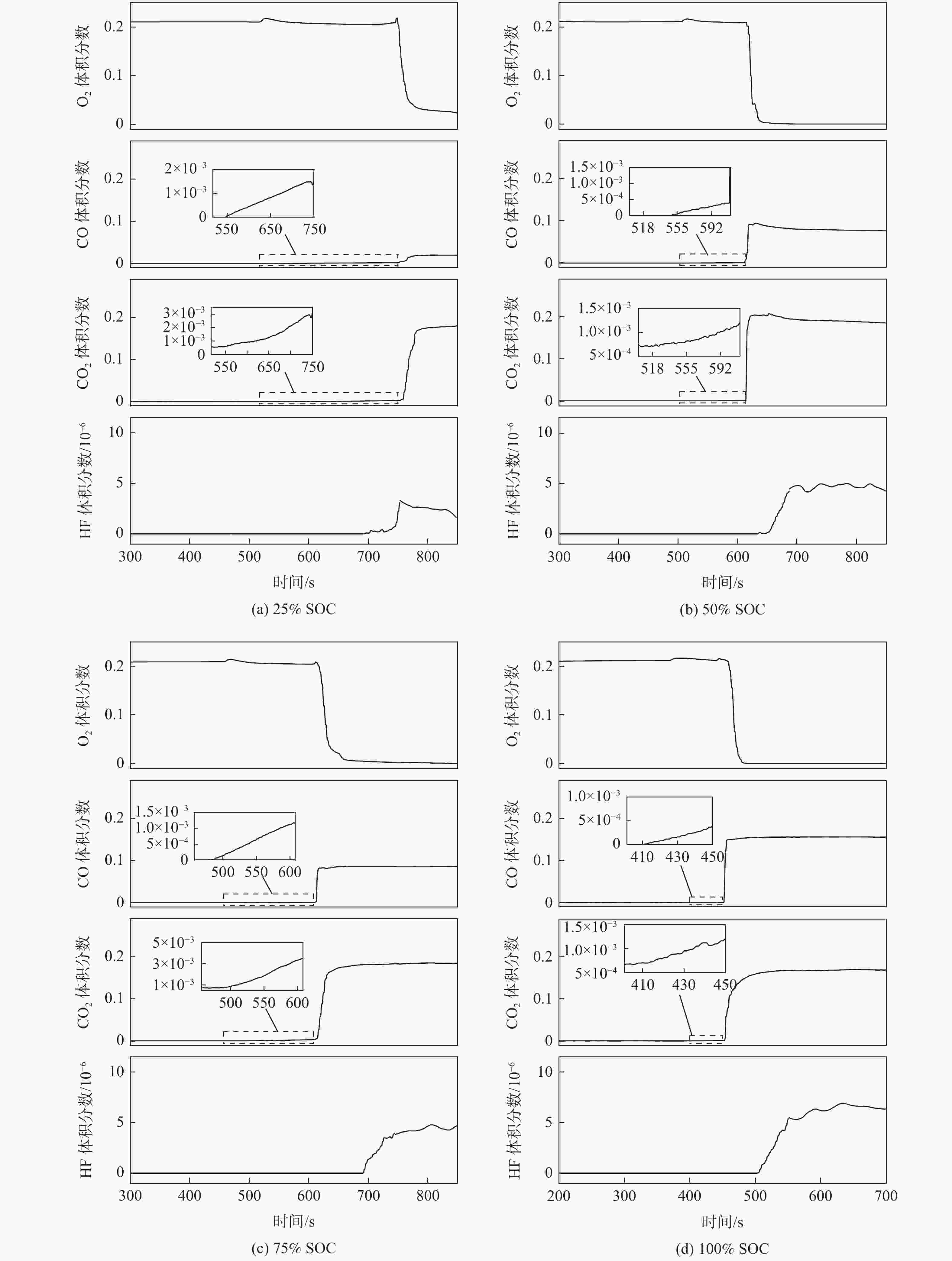
SOC n0/mol nTR/mol Δn/mol ΔV/L 25% SOC 0.838 0.855 0.017 0.381 50% SOC 0.834 0.928 0.094 2.106 75% SOC 0.836 1.081 0.245 5.488 100% SOC 0.838 1.112 0.274 6.138 表 4 不同SOC电池释放毒性气体体积分数
Table 4. Concentration of toxic gas released by batteries at different SOC
SOC VHF/10−6 VCO $V_{{\mathrm{CO}}_2} $ 25% SOC 2.85 0.0198 0.1793 50% SOC 4.74 0.0791 0.1922 75% SOC 4.33 0.0865 0.1832 100% SOC 6.29 0.1557 0.1680 注:CO的IDLH限值为0.012,CO2的IDLH限值为0.04,HF的IDLH限值为0.3。 表 5 不同SOC电池热失控气体毒性风险率
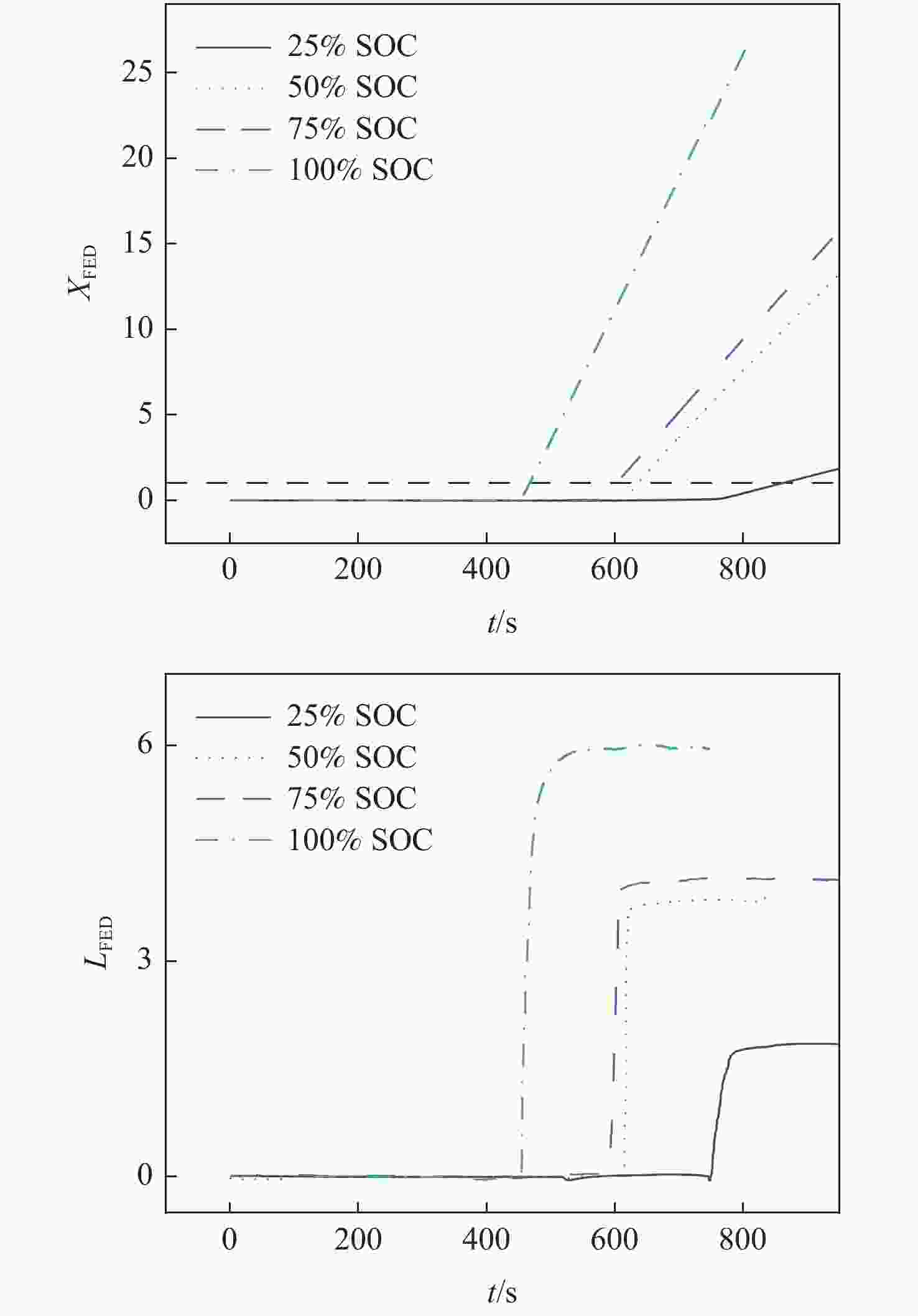
Table 5. Risk rate of thermal runaway gas toxicity of different SOC batteries
SOC I LFED $t_{{{X}}_{\mathrm{FED}}=1} $/s V 25% SOC 2.86 1.85 29 0.022 50% SOC 2.72 3.85 20 0.071 75% SOC 2.66 4.15 19 0.082 100% SOC 2.53 5.97 14 0.169 -
[1] 齐创, 邝男男, 张亚军, 等. 高比能锂离子电池模组热扩散行为仿真研究[J]. 高电压技术, 2021, 47(7): 2633-2643. doi: 10.13336/j.1003-6520.hve.20201049QI C, KUANG N N, ZHANG Y J, et al. Simulation study on the thermal propagation behavior of high energy density lithium-ion battery module[J]. High Voltage Engineering, 2021, 47(7): 2633-2643(in Chinese). doi: 10.13336/j.1003-6520.hve.20201049 [2] 张青松, 曲奕润, 郝朝龙, 等. 三元锂离子电池热失控气体原位分析[J]. 高电压技术, 2022, 48(7): 2817-2825.ZHANG Q S, QU Y R, HAO C L, et al. In-situ analysis of thermal runaway gas in ternary lithium-ion battery[J]. High Voltage Engineering, 2022, 48(7): 2817-2825(in Chinese). [3] 张青松, 刘添添, 郝朝龙, 等. 锂离子电池热失控气体快速检测及危险性方法[J]. 北京航空航天大学学报, 2023, 49(9): 2227-2233.ZHANG Q S, LIU T T, HAO C L, et al. Study on rapid analysis method of thermal runaway gas composition and risk of lithium ion battery[J]. Journal of Beijing University of Aeronautics and Astronautics, 2023, 49(9): 2227-2233(in Chinese). [4] 张青松, 罗星娜, 程相静, 等. 基于锂离子电池温降指数的细水雾添加剂筛选方法[J]. 北京航空航天大学学报, 2020, 46(6): 1073-1079. doi: 10.13700/j.bh.1001-5965.2019.0362ZHANG Q S, LUO X N, CHENG X J, et al. Method for screening fine water mist additive based on temperature drop index of lithium-ion battery[J]. Journal of Beijing University of Aeronautics and Astronautics, 2020, 46(6): 1073-1079(in Chinese). doi: 10.13700/j.bh.1001-5965.2019.0362 [5] 张青松, 刘添添, 赵子恒. 锂离子电池热失控气体燃烧对热失控传播影响的量化方法研究[J]. 北京航空航天大学学报, 2023, 49(1): 17-22.ZHANG Q S, LIU T T, ZHAO Z H. Study on quantitative method of the influence of thermal runaway gas combustion on thermal runaway propagation of lithium ion battery[J]. Journal of Beijing University of Aeronautics and Astronautics, 2023, 49(1): 17-22(in Chinese). [6] SAID A O, LEE C, STOLIAROVS I. Experimental investigation of cascading failure in 18650 lithium ion cell arrays: Impact of cathode chemistry[J]. Journal of Power Sources, 2020, 446: 227347. doi: 10.1016/j.jpowsour.2019.227347 [7] GAO S, LU L, OUYANG M, et al. Experimental study on module-to-module thermal runaway-propagation in a battery pack[J]. Journal of the Electrochemical Society, 2019, 166(10): A2065-A2073. doi: 10.1149/2.1011910jes [8] FENG X, SUN J, OUYANG M, et al. Characterization of penetration induced thermal runaway propagation process within a large format lithium ion battery module[J]. Journal of Power Sources, 2015, 275: 261-273. [9] LARSSON F, ANDERSSON P, BLOMQVIST P, et al. Author correction: Toxic fluoride gas emissions from lithium-ion battery fires[J]. Scientific Reports, 2018, 8(1): 5265. [10] LAMB J, ORENDORFF C J, ROTH E P, et al. Studies on the thermal breakdown of common Li-ion battery electrolyte components[J]. Journal of the Electrochemical Society, 2015, 162(10): A2131-A2135. doi: 10.1149/2.0651510jes [11] GOLUBKOV A W, FUCHS D, WAGNER J, et al. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes[J]. RSC Advances, 2014, 4(7): 3633. doi: 10.1039/C3RA45748F [12] ABRAHAM D P, ROTH E P, KOSTECKI R, et al. Diagnostic examination of thermally abused high-power lithium-ion cells[J]. Journal of Power Sources, 2006, 161(1): 648-657. [13] SUN J, LI J, ZHOU T, et al. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery[J]. Nano Energy, 2016, 27: 313-319. [14] LEBEDEVA N P, BOON-BRETT L. Considerations on the chemical toxicity of contemporary Li-ion battery electrolytes and their components[J]. Journal of the Electrochemical Society, 2016, 163(6): A821-A830. [15] NEDJALKOV A, MEYER J, KOHRING M, et al. Toxic gas emissions from damaged lithium ion batteries—Analysis and safety enhancement solution[J]. Batteries, 2016, 2(1): 5. doi: 10.3390/batteries2010005 [16] STURK D, ROSELL L, BLOMQVIST P, et al. Analysis of Li-ion battery gases vented in an inert atmosphere thermal test chamber[J]. Batteries, 2019, 5(3): 61. [17] National Research Council. Acute exposure guideline levels for selected airborne chemicals (Volume 13)[M]. Washington, D.C.: National Academies Press, 2013. [18] LIU P J, LI Y Q, MAO B B, et al. Experimental study on thermal runaway and fire behaviors of large format lithium iron phosphate battery[J]. Applied Thermal Engineering, 2021(1): 1-13. [19] PARK Y U, SEO D H, KIM B, et al. Tailoring a fluorophosphate as a novel 4 V cathode for lithium-ion batteries[J]. Scientific Reports, 2012, 2: 704. [20] LARSSON F, BERTILSSON S, FURLANI M, et al. Gas explosions and thermal runaways during external heating abuse of commercial lithium-ion graphite-LiCoO2 cells at different levels of ageing[J]. Journal of Power Sources, 2018, 373: 220-231. [21] RIBIÈRE P, GRUGEON S, MORCRETTE M, et al. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry[J]. Energy & Environmental Science, 2012, 5(1): 5271-5280. [22] DIAZ F, WANG Y, WEYHE R, et al. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries[J]. Waste Management, 2019, 84: 102-111. [23] 张宇, 白伟, 史砚磊, 等. 基于热失控风险指数的锂电池安全评价方法[J]. 北京航空航天大学学报, 2021, 47(5): 912-918.ZHANG Y, BAI W, SHI Y L, et al. Evaluation method of lithium battery safety based on thermal runaway risk index[J]. Journal of Beijing University of Aeronautics and Astronautics, 2021, 47(5): 912-918(in Chinese). [24] International Organization for Standardization. Life-threatening components of fire-guidelines for the estimation of time to compromised tenability in fires: ISO 13571[S]. Geneve: International Organization for Standardization, 2012. [25] International Organization for Standardization. Estimation of the lethal toxic potency of fire effluents: ISO 13344[S]. Geneve: International Organization for Standardization, 2015. [26] 王青松, 平平, 孙金华. 锂离子电池热危险性及安全对[M]. 北京: 科学出版社, 2017: 123-124.WANG Q S, PING P, SUN J H. Thermal hazards and safety measures of lithium-ion batteries[M]. Beijing: Science Press, 2017: 123-124(in Chinese). [27] YANG H, ZHUANG G V, ROSS JR P N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6[J]. Journal of Power Sources, 2006, 161(1): 573-579. [28] KAWAMURA T, OKADA S, YAMAKI J I. Decomposition reaction of LiPF6-based electrolytes for lithium ion-cells[J]. Power Sources, 2006, 156(2): 547-554. doi: 10.1016/j.jpowsour.2005.05.084 [29] WILKEN S, TRESKOW M, SCHEERS J, et al. Initial stages of thermal decomposition of LiPF6-based lithium ion battery electrolytes by detailed Raman and NMR spectroscopy[J]. RSC Advances, 2013, 3(37): 16359-16364. [30] TIKUISIS P, KANE D, MCLELLAN T, et al. Rate of formation of carboxyhemoglobin in exercising humans exposed to carbon monoxide[J]. Journal of Applied Physiology, 1992, 72(4): 1311-1319. doi: 10.1152/jappl.1992.72.4.1311 -







 下载:
下载: